Part:BBa_K1701001
PbrR
A lead-specific binding protein, PbrR, and promoter pbr from the lead resistance operon, pbr, of Cupriavidus metallidurans CH34 have shown highly sensitive and selective whole-cell detection of lead ions.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal PstI site found at 376
- 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 376
- 21COMPATIBLE WITH RFC[21]
- 23INCOMPATIBLE WITH RFC[23]Illegal PstI site found at 376
- 25INCOMPATIBLE WITH RFC[25]Illegal PstI site found at 376
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 167
Background
Certain living organisms usually have mechanisms for maintaining homeostasis and resistance to heavy metal ions including lead. This offered an opportunity for genetically engineered microorganisms applied to lead detection and absorption. Taking advantage of a Pb2+ regulatory metalloprotein from Cupriavidus metallidurans CH34, He et al. developed a fluorescent biosensor with high selectivity and sensitivity to Pb2+ ions. Recently, Jouanneau et al. reported the identification of four heavy metals, including lead in environmental samples using bioluminescent bacteria.
Biology
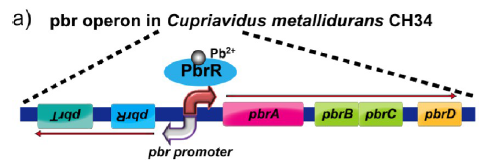
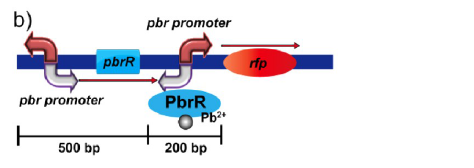
A lead-specific binding protein, PbrR, and promoter pbr from the lead resistance operon, pbr, of Cupriavidus metallidurans CH34 have shown highly sensitive and selective whole-cell detection of lead ions. The display of PbrR on the E. coli cell surface permitted selective adsorption of lead ions from solution containing various heavy metal ions. The following figure shows the genetic organization of the pbr operon locus in the C. metallidurans CH34 genome.
Figure 1(a).Genetic organization of the pbr operon locus in the C. metallidurans CH34 genome.
Figure 1(b).Genetic organization of the lead inductive RFP expression plasmid in E. coli.
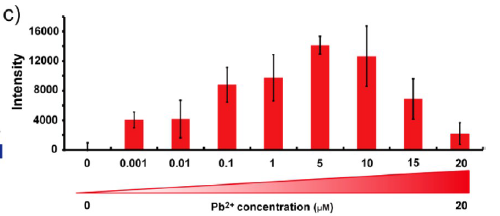
Figure 1(c). Fluorescence measurement of E. coli cells containing the lead induced RFP expression plasmid after gradient concentrations of Pb2+ induction and resuspended in PBS buffer (pH 7.4). All of the cell numbers were normalized to 3 × 107 before the tests.
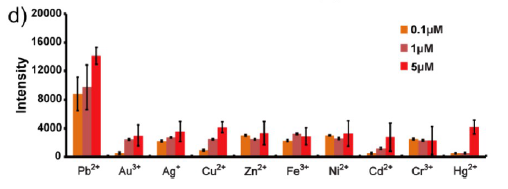
Figure 1(d). Fluorescence measurement of E. coli cells containing the lead induced RFP expression plasmid with concentrations of 0.1, 1, and 5 μM each metal ions. All of the cell numbers were normalized to 3 × 107 before the tests.
Protein sturcture
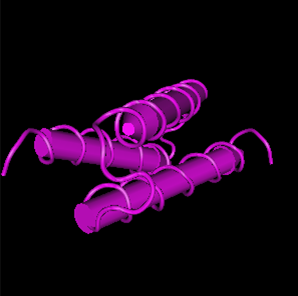
Figure 2. The 3D structure of protein of PbrR. From https://www.uniprot.org/uniprotkb/Q58AJ5/entry
Part uses
we heterologously expressed the lead-binding chaperon PbrR gene from C. metallidurans into Bacillus subtilis so as to present a selective lead-sensing system and a remediation system respectively using the gene elements of the lead-specific operon pbr, including the lead binding protein PbrR from C. metallidurans CH34. In the subsequent engineering, we displayed PbrR on Bacillus subtilis biofilm or its spore surface to construct a whole-cell absorption specific for lead, which is able to be easily recovered and therefore is good for application outside of laboratory settings. What’s more, it can adsorb lead under both benign conditions and adverse circumstances.
We used TasA, the major protein of the biofilm matrix component in B. subtilis, and CotC, the B.subtilis spore component, as fusion partner to display PbrR.
When nutrition is sufficient, we can use plant polysaccharides to induce the formation of biofilm, on which protein TasA can be simultaneously expressed with PbrR. Besides, when conditions get adverse for the growth of cells, B. subtilis produce spores to survive. If we express the PbrR along with the coat protein CotC, spores will be capable of lead adsorption. Therefore, B. subtilis can adsorb lead under both benign conditions and adverse circumstances, which tremendously improve the efficiency of lead adsorption.
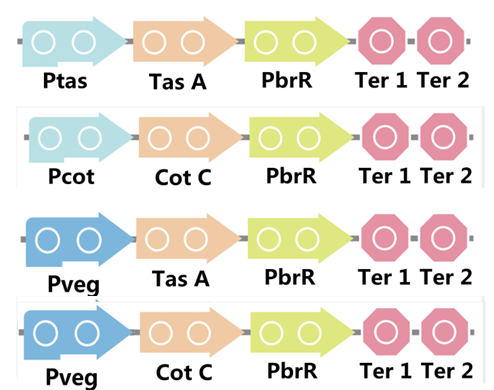
To realize this goal, we have designed four pathways shown in Figure 3, which are used in our project. In order to improve the efficiency of the expression of the two former pathways, we utilized a constitutive promoter Pveg, a biobrick part in the biological parts of iGEM used by us to replace the promoter Ptas and Pcot, which is involved in the two latter payways.
Figure 3. Four pathways designed by our group involving PbrR part.
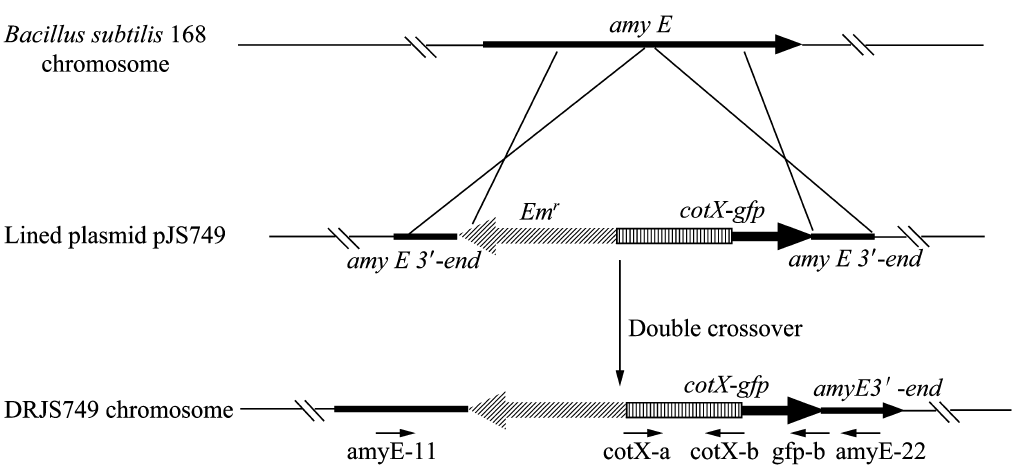
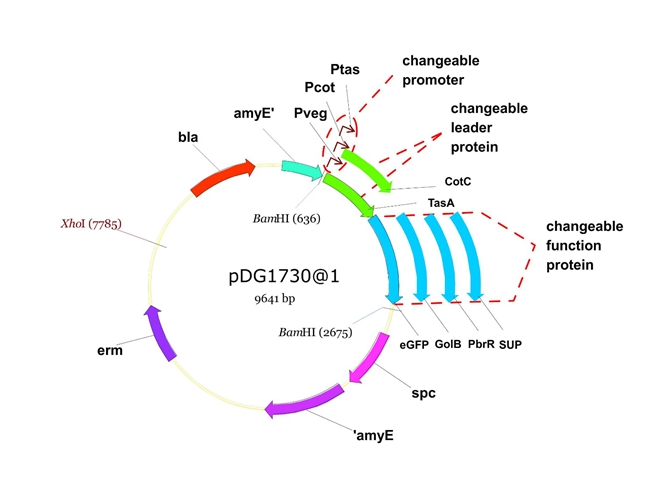
We will construct our plasmid in E.coli first and affirm the feasibility of the pathways, after which we will transform the plasmid into B.subtilis. After linearization of the plasmid, we can integrate the target genes into the chromosomes of B.subtilis utilizing homologous recombination. The process and the shuttle plasmid of our project can be shown as follows.
Figure 4. The process to integrate the target genes into the chromosomes of B.subtilis utilizing homologous recombination.
Figure 5. The shuttle plasmid designed by our project including the preceding pathways.
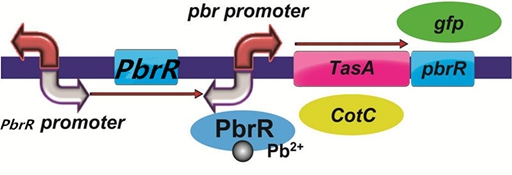
To improve the selectivity of lead adsorption, we had a plan to design a pathway as follows. With the specific promoter pbr promoter, the heavy metal-binding biofilm protein/coat protein can be expressed only when there is specific lead in the solution. In addition, we could do a pre-test to demonstrate the feasibility of the pathway by replacing PbrR with the reporter protein GFP.
Figure 6. The designed pathway to improve the selectivity of lead adsorption.
Results
Homologous recombination
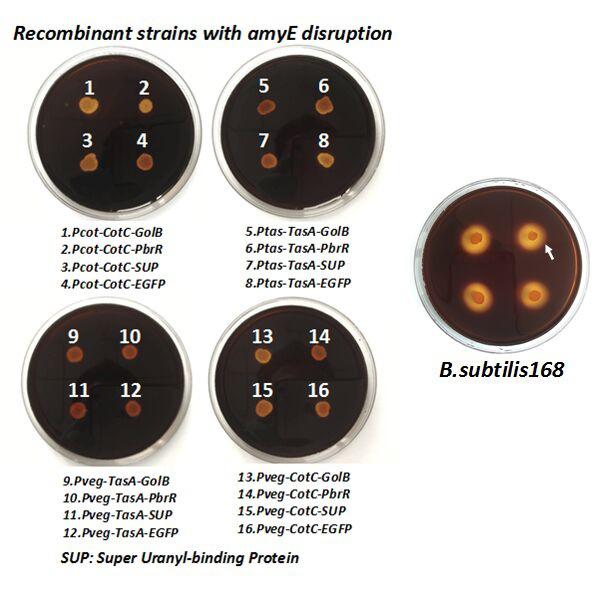
After we constructed the 16 kits in E.coli, we inserted them into the shuttle plasmid pDG1730 which can transfer from E.coli to B. subtilis. We insert our fusion genes between two sequences of the amylase genes on the plasmid. By homologous recombination, our fusion genes will replace the amylase gene on the genome of B. subtilis. In order to test and verify whether homologous recombination is successful, we built the following three steps. Antibiotic selection, bacterial PCR analysis and amylase activity analysis. After a series of test and verification experiments, we have successfully inserted the 16 kits into the genome of B. subtilis. The following figure shows the result of the amylase activity analysis. The picture on the right is the control group while the pictures on the left are the experiment groups. When we applied iodine on the plate with starch, there would be hydrolysis cycles on the plate because the α-amylase gene wasn’t replaced. However, there wouldn’t be hydrolysis cycles on the plate if homologous recombination was successful.
Figure 7. Result of homologous recombination.
Bio-adsorption
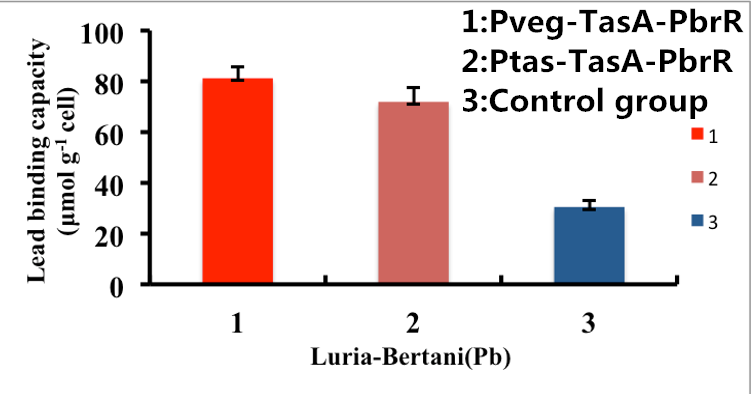
As shown in Fig. 8, B. subtilis bacteria with the TasA-dependent surface displayed PbrR with promoter Pveg were able to adsorb lead ions with a capacity of about 81.2 μmol g-1cells, which is 3-fold higher than un-displayed samples. While Bacteria with promoter Ptas were able to adsorb lead ions with a capacity of about 69.5 μmol g-1cells, which is 2-fold higher than un-displayed samples.
Figure 8. Adsorption of lead ions by B. subtilis TasA-dependent surface-displayed PbrR protein.
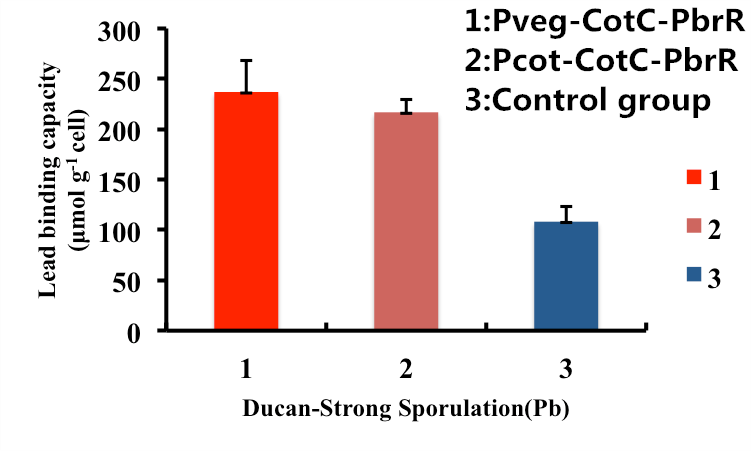
As shown in Fig. 9, B. subtilis endospores with the CotC-dependent surface displayed PbrR with promoter Pveg were able to adsorb lead ions with a capacity of about 227.6 μmol g-1cells, which is 2-fold higher than un-displayed samples. While bacteria with promoter Pcot were able to adsorb lead ions with a capacity of about 225.1 μmol g-1cells, which is 2-fold higher than un-displayed samples.
Figure 9. Adsorption of lead ions by B. subtilis endospore CotC dependent surface-displayed PbrR protein.
In conclusion, the engineered B. subtilis as well as the endospores worked well in heavy metal adsorption. Besides, it is proved by us that Pveg is a more efficient promoter than Ptas and Pcot, so maybe we can utilize promoter Pveg when engineering B. sublitis.
Improvement for Lead Recovery
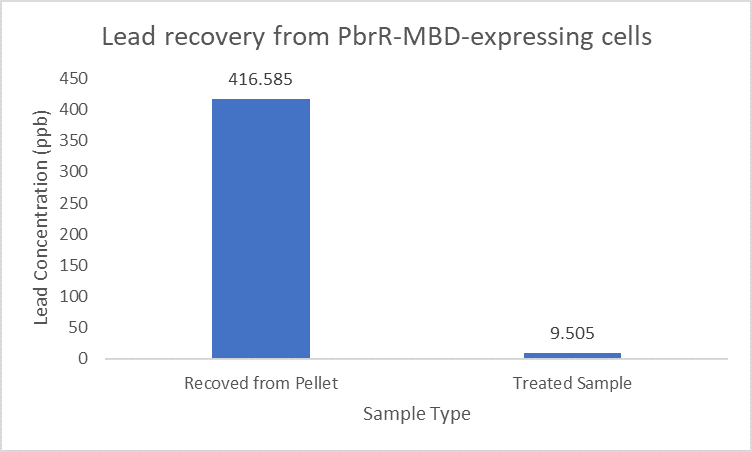
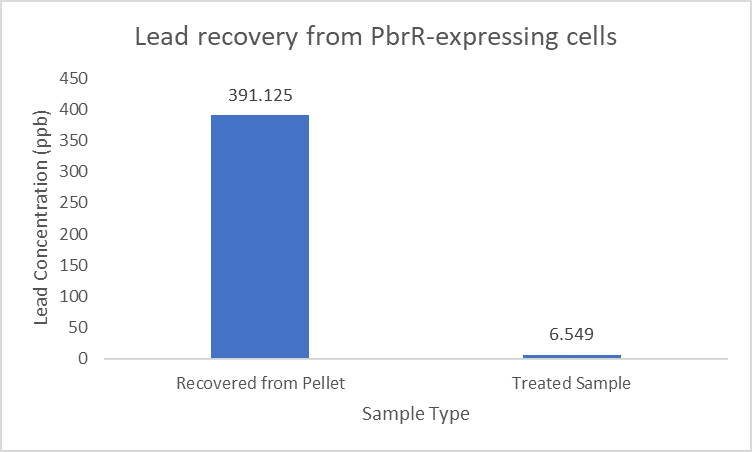
PbrR was modified by truncating the N-terminal DNA Binding Domain and C-terminal redundant amino acid residues to obtain lead domain maintaining its Pb2+ binding property. The MBD (metal binding domain) (Part:BBa_K4428001) being smaller in size leads to increased display and expression than the full-length PbrR-displayed cells.
Importantly, the Pb2+ adsorption capacity of PbrR MBD-displayed cells was about 1.92-fold higher than that of the full-length PbrR-displayed cells. Also, the PbrR MBD shows highly selective adsorption towards the lead similar to the full-length PbrR (Hui et al. 2018).
When subject to the same 400 ppb concentration of lead, PbrR MBD is able to remove as much amount of lead from the system as PbrR. This clearly suggests that even this smaller protein is sufficient for lead adsorption at 0.4 mg/l concentration of lead which is the concentration of lead in industrial effluents. ICP-MS results suggest that this is nowhere near the maximum capacity of adsorption of the proteins which would actually be much more than what is currently tested. However, this in itself suggests that even this smaller protein can work as well as PbrR in these concentrations and thus, reduces the metabolic burden of the cell.
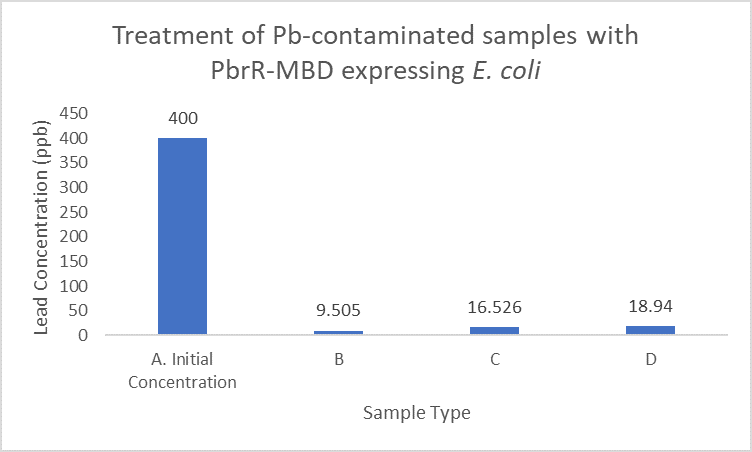
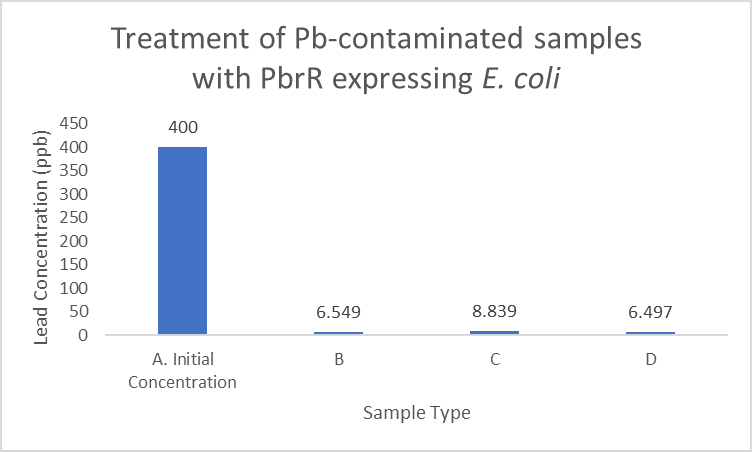
Further, it observe that a greater amount of lead is successfully desorbed from the cells expressing PbrR-MBD on their surface than PbrR. This suggests that PbrR-MBD may have an even greater ability to recover lead than PbrR. This observation holds great significance when thinking about the recycling of lead instead of just its removal.
References
[1].Wei, W., et al., Simple whole-cell biodetection and bioremediation of heavy metals based on an engineered lead-specific operon. Environ Sci Technol, 2014. 48(6): p. 3363-71.
//chassis/prokaryote/bsubtilis
| biology | Cupriavidus metallidurans CH34 |