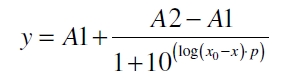Part:BBa_K389015:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K389015
Transfer function
The data for the transfer function was measured and analyzed as described below. The data was fitted with a dose response function of the form
with the Hill coefficient p, the bottom asymptote A1, the top asymptote A2 and the switch point log(x0). Figure 1 shows the measured normalized specific production rates qP,n plotted against the logarithm of the concentration of the inductor acetosyringone in µM. The fit has an R2 = 0.98.
The important data from the transfer function is summarized in table 1:
Table 1: Data from the transfer function for the part BBa_K389015.
| Parameter | Value |
|---|---|
| Hill coefficient | 1.092 |
| Switch point | 31.6 µM |
| Top asymptote | 2.16 |
The fully induced VirA/G signaling system with luciferase read-out has a 2.2 fold increased expression compared to the uninduced system. The Hill coefficient is > 1, so a positive cooperation can be observed ([http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WMD-4V42JG5-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=b6431553217aca1129c5b441f4b78425 D Chu et al., 2009]). The switch point of the system is at about 32 µM, so this is the concentration at which the device output is 50% of the maximum output.
Response time
The system needs at least one hour to show a measurable reaction to an induction with acetosyringone. In the following illustration the reaction of the system to induction with 200 µM acetosyringone in the exponential growth phase is shown. For a good separation of the induced system from the uninduced system at least two hours are needed.
Data Analysis
Because the luciferase accumulation is very different in different cultivations, the uninduced negative control was used as internal standard. To show the behaviour of the VirA/G signaling system when induced, the ratio ɸI between induced (i) and uninduced (u) relative luminescence unit (RLU) per OD600 is calculated:
As seen above, at least one hour is needed to separate the induced luminescence signal from the uninduced, so ɸI > 1. Within a cultivation ɸI is rising during the first hours and is decreasing after it reached a maximum at OD600 ~ 1. This is shown in figure 3:

To measure the ratio of increasing promoter activity by inducing the system ɸI samples for analyzation should be taken at OD600 ~ 1.
Protocols
Cultivation
- Inoculate 10 mL LB containing desired Antibiotic with glycerol stock
- Cultivate over night at 37 °C and 175 rpm
- Measure the OD600
- Prepare shake flasks with LB, antibiotic and inductor
- For luciferase measurement at least 10 mL starting volume
- Inoculate the main culture with a starting OD600 of 0.1
- Cultivate at 37 °C and 175 rpm
- Take a sample at least every hour and measure the OD600
Measurement
- For the luciferase detection we used a [http://www.promega.com/tbs/tb281/tb281.pdf Promega Luciferase Assay System], containing a Cell Culture Lysis Reagent, Luciferase Assay Substrate and Luciferase Assay Buffer
- Prepare reaction tubes with 10 µL of high salt buffer (1M K2HPO4, 20mM EDTA, pH 7.8)
- Add 90 µL sample, mix and freeze at -80 °C
- For the measurement thaw by placing the tubes in room temperature water
- Add 300 µL of freshly prepared lysis mix ( 1X Cell Culture Lysis Reagent, 1.25 mg/mL lysozyme, 2.5 mg/mL BSA, add water for desired volume)
- Mix and incubate the cells for 10 minutes at room temperature
- Prepare the Luciferase Assay Reagent, by adding 10 mL of Luciferase Assay Buffer to the vial containing the Luciferase Assay Substrate
- Fill each well of a white, flat bottom 96 well microtiter plate with 20 µL of cell lysate
- For the detection of luciferase use a plate reading luminometer with injector for the Luciferase Assay Reagent and following settings (we used a Promega GloMax®-Multi Detection System with dual injector):
- Injection volume of Luciferase Assay Reagent: 100 µL
- Delay: 20 secs
- Integration: 3 secs
References
Chu D, Zabet NR, Mitavskiy B (2009) [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WMD-4V42JG5-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&_docanchor=&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=b6431553217aca1129c5b441f4b78425 Models of transcription factor binding: Sensitivity of activation functions to model assumptions], J Theor Biol 257(3):419-429.
User Reviews
UNIQ24d694437ee76a3f-partinfo-00000005-QINU UNIQ24d694437ee76a3f-partinfo-00000006-QINU