Help:Spring 2010 DNA distribution
This year at iGEM we have made a few important changes to the Registry and the DNA distribution. The Spring 2010 Distribution uses the same 384 well format used in 2007 and 2009, along with our extensive quality control measures. While we will not be sending out all of the parts in the Registry, we have taken care to create a kit that includes both high-quality parts from the past as well as new parts from 2009 that were designated by teams as favorites. With a smaller number of confirmed quality controlled parts, (3) 384 well plates total, the Spring 2010 Distribution Kit will be a very effective basis for your synthetic biology projects.
Additionally this year we have also included plasmid backbones for three of our standard plasmids. The linearized plasmid backbones for pSB1C3, pSB1T3, and pSB1A3 are pre-cut at the EcoRI and Pst1 sites and are.
Whats included with the Spring 2010 Distribution
- Spring 2010 DNA Distribution (3) 384 well plates
- Linearized Plasmid Backbones for pSB1C3, pSB1T3, and pSB1A3
- iGEM Stickers and Pins!
Getting Started with the Spring 2010 DNA Distribution
Storage
Since the distribution kit is comprised of dried dna, it is quite stable for storage at room temperature. However once 10ul of diH20 are added to any of the wells, we recommend either storing the kit plate with its plastic cover in a -20C freezer, or aspirating the rest of the resuspended dna from the well and keeping it separately in a -20C freezer.
Note that there is not enough DNA to perform anything but transformations from the DNA in the wells. See the Usage section below for more information.
The linearized plasmid backbones should be stored at 4C.
Usage
The Spring 2010 DNA Distribution is a very useful tool available to you to perform synthetic biology with standard biological parts. It contains dry DNA of hundreds of parts that are available in the Registry up to 2009. You will transform the DNA into cells and make your own glycerol stocks of any part that you wish. However it does not contain enough DNA to do assembly. To use the DNA in the Distribution Kit you may follow these instructions:
- With a pipette tip, punch a hole through the foil cover into the corresponding well to the Biobrick™-standard part that you want. Make sure you have properly oriented the plate. We recommend that you do not remove the foil cover, as it could lead to cross contamination between the wells.
- Add 10uL of diH2O (deionized water)
- Pipette 1 or 2uL of the resuspended DNA transform into your desired competent cells, plate bacteria with the appropriate antibiotic* and grow overnight.
- Pick a single colony and inoculate broth (again, with the correct antibiotic) and grow for 18 hours.
- Use the resulting culture to [http://openwetware.org/wiki/Miniprep/Kit-free_high-throughput_protocol miniprep] the DNA AND make your own glycerol stock (for further instruction on making a glycerol see [http://openwetware.org/wiki/Endy:Making_a_long_term_stock_of_bacteria this page]).
*To know which antibiotics to use, look at the plasmid that the part is in. The naming scheme for plasmids is specifically designed to indicate antibiotic resistance.
Choosing Parts
On the left-hand side of the Registry Main Page you will find a Catalog of Parts and Devices. This tool is meant to assist in the synthetic process through several avenues.
- It allows users to locate parts based on their function.
- It gives users the freedom to browse through different devices by type, through a series of tables which link to information in the parts registry.
- Parts and devices can be browsed by chassis. Unless otherwise specified, most parts in the Registry work in Escherichia coli.
- Parts and devices can also be browsed by standard. Unless otherwise specified, most parts in the Registry comply with the original BioBrick assembly standard (also known as Assembly standard 10).
- Parts and devices can be browsed by contributor.
- The chassises can be browsed to locate a particular strain.
Locating a Part in the Distribution
Prior to taking the DNA Distribution Kit out for use, it is helpful to know both the location of the part(s) you want, as well as the plasmid and antibiotic resistance(s) of the required parts. The Registry provides the necessary tools to locate your desired parts within the 384 well plates used in the distribution, along with antibiotic resistance and other pertinent information. If you would like to find a part to serve a particular function but have not yet picked it out, you can locate various parts by browsing, as described below. If, on the other hand, you already have a specific part number in mind, you can find its location by Focused Search.
Browsing
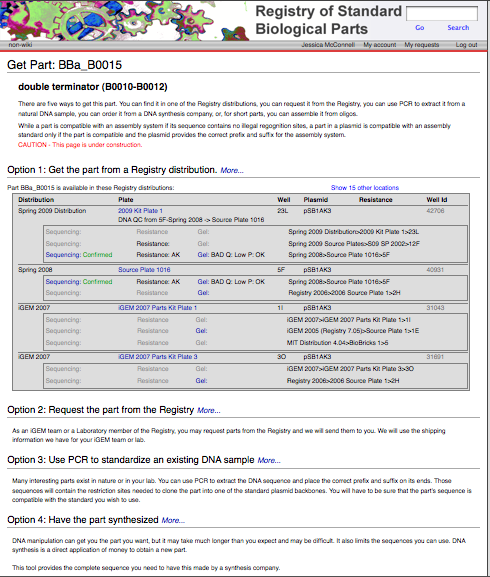
After choosing a particular family of parts in the Catalog, users can select the link identifying that group of parts (i.e., Promoters) to view an explanation of the general structure and function of those parts, as well as information to help better design and document new variations on the parts, and a help section featuring further reading materials. From that introductory page, it is possible to use sorting mechanisms in the Catalog section (for example, categorizing promoters by associated RNA polymerase and by regulatory mechanism) to narrow down the search until arriving at a listing of the desired parts in the registry (for example: Positively regulated E. coli σ70 promoters). These parts have a green box filled by an "A" to the left of the row if they are available. At this point, by selecting the name of the part (Ex: BBa_I1051) it is possible to view further description of the part's features; clicking on the link (Get This Part) at the top right portion of the screen will yield the plate and well location of the part in the Spring 2009 parts distribution.
Focused Search
- Go to the Registry.
- In the search box on the upper right, enter the part number that you are interested in (e.g. BBa_B0015) and click GO.
- Click on the Get This Part link at the top right part of the page. Please note that we only have physical DNA for parts whose part status (in the box located just above the Get This Part link) reads Available.
- By following the Get This Part link, you will arrive at a page outlining the location of the part in the parts kit (Spring 2010 distribution), along with QC information and some other ways of obtaining the part if it is not included in this year's parts kit.
Distribution plate orientation
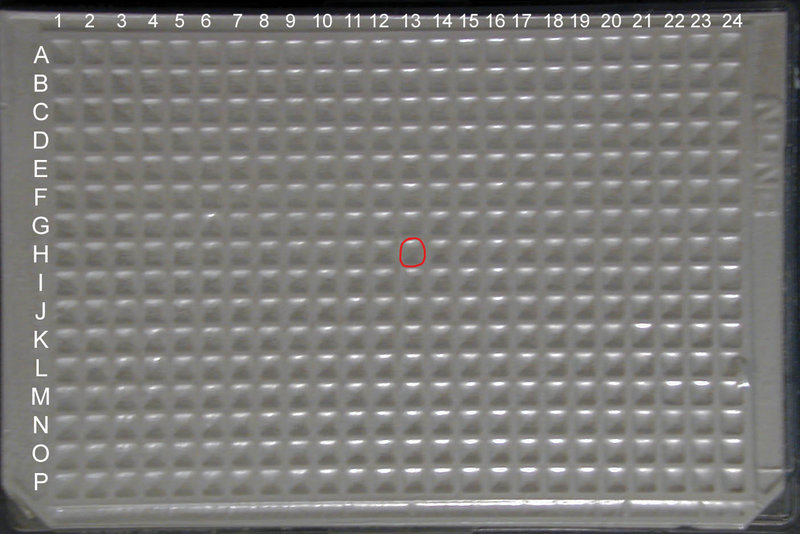
For the Spring 2010 distribution, you will receive a set of (3) 384 well plates, from which you can extract the part of your choice once you have located it in the kit. Unfortunately, the foil cover will obscure both column and well markings. However, you can still find your part by correctly orienting the plate using the two notched corners as markers: well A1 is located at the upper left corner of the plate when the long side of the plate with the notched corners is considered the bottom.
Once you have found the well which your BioBrick™ part of choice is located in by searching for it through the Registry (for example well 13H in iGEM 2009 DNA Parts Kit Plate 1) you want to count across the plate starting with Column 1 until you get to Column 13 and down the plate starting with Row A until you get to Row H.
MAKE SURE that the two notched corners of the plate are oriented at the BOTTOM of the plate (see the top view image at left for correct orientation)
Availability
If you decide to do a targeted search for a particular part that you think you might want to use, you need to make sure that the part is actually available. There are many parts in the Registry that people are still working on, or decided not to continue working on anymore, therefore we never received or don't yet have the physical DNA for them. This of course means that the DNA is not available in 2009 DNA Parts Kit. The simplest way to tell whether the part is available is to look at the top right of the part's Main Page. If the part is available the top part of the box will be green and say "DNA available."
Ordering a part
We've taken care to create a very well-rounded DNA Parts Kit this year, including the high quality parts from previous years, and the 2008 parts deemed as favorites by all of the teams. However, should you find the part, or parts, that you require are not available in the distribution but available in the Registry, feel free to send us an email (hq [AT] igem [DOT] org) in order to request it. Just include the part name, the plasmid it's located in, and the source, and we'll send it out to you
Quality Control Process
For this year's distribution we have carried over our extensive quality control measures conducted in 2008, in order to better ensure the accuracy of DNA parts sent to the iGEM 2009 teams. The QC process from 2008 examined each part in the Registry to determine if the plasmid and the part within are correct according to the documentation provided with each submission. The results for each part were then posted on the Registry's DNA repository page, allowing iGEM teams to access this information before using the submissions to create their own parts. This year's DNA Parts Kits contains hundreds of these high quality parts from previous years, as well as the team favorites from 2008, which have all undergone the same quality control measures before inclusion into the 2009 kit.
Streaking for Single Colonies
Once we decided which part would be assigned to each plate and created the full layout for the distribution, bacterial stocks containing the parts were streaked for single colonies on petri dishes containing the appropriate antibiotic.
Parts in the Registry prior to iGEM 2008, that had all undergone an extensive quality control process in 2008, were kept in glycerol stocks in the Registry's -80C freezer, where they were directly streaked onto petri dishes with appropriate antibiotic and grown overnight at 37C.
The rest of the parts used for our distribution - the favorites of the iGEM 2008 teams - were received as DNA and transformed into competent cells. Once transformed, they were also plated onto petri dishes with their appropriate antibiotic and allowed to incubate overnight at 37C.
Preparation and Inoculation from Staging Plate
The next morning (16 hours of incubation), the petri dishes were removed from the incubator and a single colony was picked from each plate, using a pipette tip. The colony was then transferred to its corresponding well of a 96 deep well plate filled with the appropriate antibiotic broth (60ul in each well), and mixed thoroughly. This series of inoculations created a 96 well staging plate for the bioparts.
Once all 96 wells of the staging plate were inoculated with single colonies, a 5.0ul pin tool replicator was used to then inoculate from the staging plate to antibiotic testing plates,miniprep plates, and glycerol plates. The plates were then incubated at 37C for 16 hours, and 10 hours for the glycerols.
Antibiotic testing plates
Four antibiotic testing plates were prepared, testing known bacterial resistance of ampicillin, kanamycin, chloramphenicol, and tetracycline. The plates were grown overnight at 37C, pelleted and examined for resistant growth in wells. The plates are then photographed and added to the DNA repository for 2009 as part of our open quality control process. For the 2009 DNA Parts Kit, this step was applied to the favorite parts of the 2008 teams, but was forgone for the high quality confirmed parts from previous years, all of which had already gone through this process last year.
Glycerol plates
As well as the antibiotic testing plates, two glycerol plates containing 10% glycerol broth and appropriate antibiotic were prepared for the long term storage of the bacterial stocks. One of these plates will remain in the Registry -80C freezer while the other will be stored offsite.
Miniprep plates
Two miniprep plates were inoculated, and incubated overnight at 37C. The following day, the plates were pelleted, and photographed for entry into the DNA repository for 2009 as part of our open quality control process. The plates underwent miniprepping, and the DNA was then resuspended in TE and combined together. The single 96 well DNA plate along with three other 96 well plates, all of which containing different parts, was used to produce the 384 well source plates, which are the basis for the 2009 distribution. However, for the favorite parts of the 2008 teams, 15.0ul of the resuspended DNA was digested and run on an electrophoresis gel to examine the size of the plasmid and part. In addition, 24.0ul was sent to the Broad Institute for DNA sequencing. The high quality parts from previous years had already gone through this process.
Using the online QC resources
Here at iGEM, we want everyone to take a look at the results of the quality control measures we've taken this year and in 2008, in order to make an informed decision when choosing a part, so we've made sure to update the online repository for the 2009 distribution kit with our quality control results.
The best way to use our quality control information is to use it on a part by part basis. As you design your project, make sure to check every part that you're interested in for its qc data.
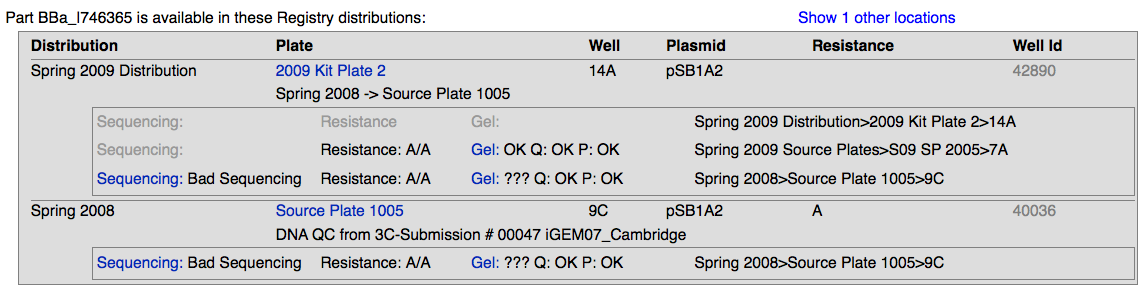
- After searching for a part in the registry and arriving at its main page, click on the "Get This Part" link which will take you to the section listing various quality control information for that part and its locations within the registry.
- There are a few different quality control ratings and details to see on this page.
Sequencing Results
The sequences are compared with their target sequence through software, and are given the following qualitative values:
- Confirmed
- Partially Confirmed
- Long Part – we don’t know whether the entire part is confirmed, but the sequence ends are
- Inconsistent
- Bad Sequence – usually caused by low DNA concentration or incorrect primers
- [U] - User Confirmed – we manually reevaluate the inconsistent sequences and look at the trace files to see if a simple shift of the sequence will confirm it
For more in-depth analysis, when reviewing quality control information for a part, just click on the "Sequence" link, right beside the part's Sequence result. Every user can look at all the results we get back from Genewiz: including the trace files, quality scores, and sequence reads.
Gel Results
We did restriction digests on parts using EcoR1 and Pst1 as our restriction enzymes. Afterwards we ran the digests on an e-gel and then imaged the gel using standardized parameters. The gel image was then uploaded to the DNA repository. We evaluated each lane, using a set of qualitative statements for plasmid length (P), plasmid quantity (Q), and insert length (Gel).
- Plasmid Quantity (P): None / LOW / OK / HIGH
- Plasmid Length/Quality (Q): OK / BAD / ???
- Insert Length/Quality (Gel): OK / BAD / ???
To view the gel results for yourself, you can click on the "Gel" link beside the gel result for your part or you can click on the "Gel Images and Results" link at the Source Plate.
Evaluating the Gel Results
On the Gel Images and Results page, you can find the expected length of the part (insert) and the plasmid, as well as the gel image. This allows you to compare the expected lengths of the part and plasmid with their gel bands.
If you find that the gel results for your part do not match with their expected lengths then you may first want to take a look at the part’s Main Page to find out its restriction sites. Some parts and plasmids may have more than one or no EcoR1 and Pst1 cut sites; which will of course differ from the expected banding on the gel. When evaluating the parts we made sure to take these exceptions into consideration; if the gel results matched the band length calculations, the part was described as OK.
Growth Plates and Antibiotic Test Plates
All source plates have images of the pelleted growths before they were sent for miniprepping, and plates 2005 and onwards also have pelleted growths on 4 antibiotic test plates. In order to view this information for your part, you should know your part's 2009 Source Plate, which you can find on the Get This Part page. From the DNA repository, find the 2009 source plate of your choice, and links to the growth and AB test plate images will be at the top.
What do AB test plates tell you?
Antibiotic resistance is measured in the resistance field, which compares the expected antibiotic resistance with the demonstrated antibiotic resistance.
- Whether a part grew up in the AB broth that its plasmid was resistant to.
- Whether the part’s plasmid is resistant to more than one AB.
If there are discrepancies between the plasmid AB description and the AB test, take a look at the gel results, to find out if the plasmid quality (P) was bad.
- If the quality (P) was BAD, the plasmid that the part is in may be incorrect. If so, check the insert length to see if the part is wrong as well.
- If the quality was OK for the plasmid, as well as the insert length, and the sequencing, then it is likely that the AB Test Plate(s) may have had an insufficient antibiotic concentration.
Parts Absent from 2009 Kit
If you find that your part of interest is not in a 2009 Source Plate or that its quality control information has not been added to the 2009 registry yet, take a look at its location on the 2008 Source Plates. If the part is not included in the 2009 distribution, first review the quality control information, and then feel free to make a request for the part to hq [AT] igem [DOT] org.