Part:BBa_K190019
fMT
[http://2009.igem.org/Team:Groningen/Project/Accumulation#fMT fMT] is a metallothionein, binding Arsenite(III) and Arsenate(V), it has higher affinity for As(III). The protein was isolated from [http://en.wikipedia.org/wiki/Fucus_vesiculosus Fucus vesiculosus] and described by Morris et al (Morris 1999). It consists of 67 amino acid residues and has 16 cysteine residues, a high cysteine content is a key feature of metallothioneins (MT). Another characteristic of MT is the lack of aromatic residues. This is also seen in fMT, as it has only one tryptophan. Two domains containing cysteine residues are presumed to be involved in the metal binding function. Unusual in fMT is the presence of a 14 amino acid linker region, between the two putative metal-binding domains, which contains no cysteine residues. fMT binds a multitude of metal ions, 6 Cd2+ ions or 5 As3+ ions in a sequential order, facilitated by the elongated linker domain (Ngu 2009).
Usage and Biology
Overexpression of fMT can be used to accumulate arsenic without toxification of the cells. For more applications see the experience page. The gene was functionally expressed in E. coli.
From literature it is known that metallothioneins are degraded inside lysozymes, especially when they are in the apo- (non-bound) state (Gold 2008), for bacteria this is not known, but from mammalian MT this can be estimated up to 0.8nmol apo-MT/mg protein/min (Klaassen 1994). This can be avoided by adding ~0.5mM metal-salts (ZnCl2, CuSo4, CdCl2) to cells expressing the protein. But before testing the accumulation of the target metal by fMT, the metals should be chelated from the metallothioneins, by for instance EDTA.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 38
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 127
- 1000COMPATIBLE WITH RFC[1000]
Results
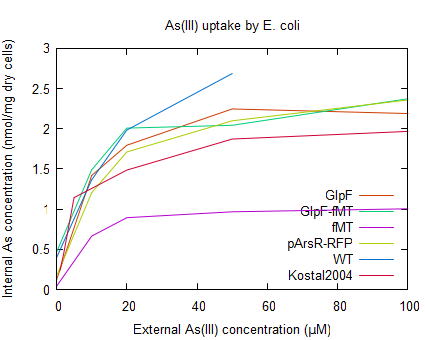
Arsenite uptake by E. coli containing pSB1A2-R0010-fMT (BBa_K190032) was tested using a arsenic uptake assay (as described by Kostal 2004) and the arsenic concentration was determined by ICP-MS (see [http://2009.igem.org/Team:Groningen/Protocols#Metal_uptake_assay_for_E._coliKostal_2004 protocols]). But because of a lack of reproducibility and a unexpected high uptake yield (as can be seen in figure 1), the functionality of fMT could not be determined. The curve of arsenic uptake in E. coli with fMT shows a lower uptake yield than the other strains, this may be caused by an accidental increase of temperature during drying the cells. For further reading on discussion of this data see the [http://2009.igem.org/Team:Groningen/Accumulation wiki].
- Figure 1: Uptake of As(III) by E. coli WT, and the strains containing the different parts of the accumulation device. As a control the arsenic uptake of E. coli with ArsR overexpression (as described by Kostal 2004) is also shown.
//cds
| function | binding of metal ions |
| n/a | fMT |