Part:BBa_K2442101:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Improvement of BBa_K2442101
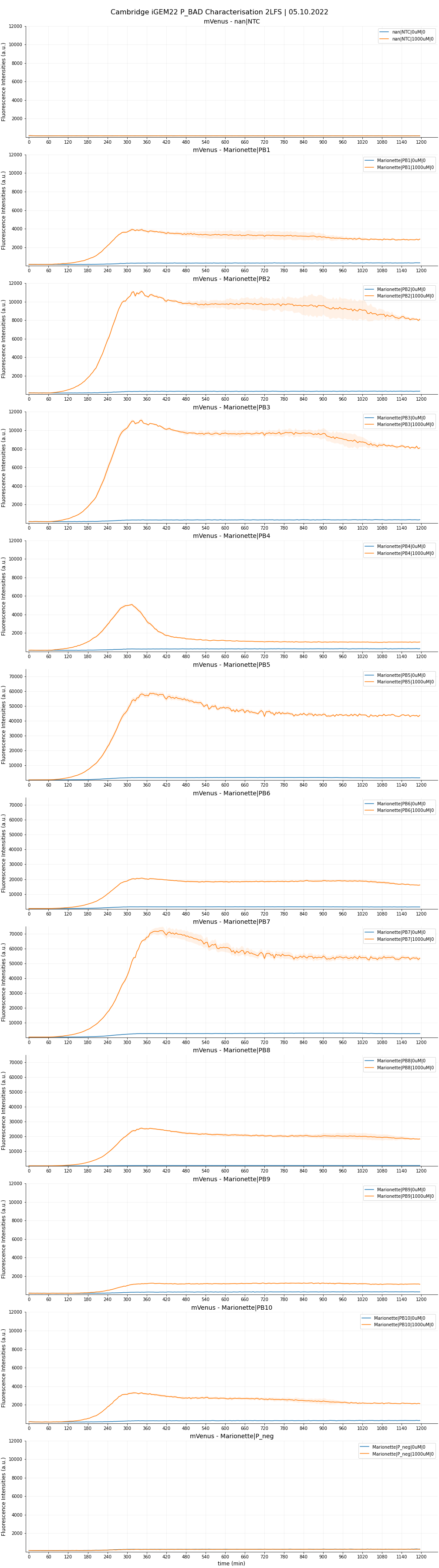
In 2022, the team at Cambridge has designed a collection of nine pBAD promoters that are all only less than 160 base-pairs - half of the wild-type BBa_K2442101's length. Four members showed certain level of improvements, with two of those (called pBAD_AP2 and pBAD_AP3) showcased very promising merits, with slightly lower leakiness but more than fourfold increase in maximal expression. Please check out their documentation entry on BBa_K4491007. Below is a brief recap of the testing result.
To test the designed araBAD promoters, we cloned nine respective Level 0 promoter parts into Level 1 JUMP constructs, using a standard Golden Gate Assembly protocol with BsaI-HFv2. Lying downstream of the promoter is a B0032 medium RBS, an mVenus reporter and a DT5 double terminator. A medium-strength RBS was chosen to avoid burden caused by accidental overexpression of mVenus. All TUs were put into a pJUMP27-1A destination vector. The low copy number plasmid makes it favourable for avoiding phototoxicity and aggregate bodies, as well as reducing noise. A list of constructs ID and their respective constituent promoter is given below.
| LVL1 ID | Promoter |
|---|---|
| PB1 | AP1 |
| PB2 | AP2 |
| PB3 | AP3 |
| PB4 | AP4 |
| PB5 | AP5 |
| PB6 | AP6 |
| PB7 | AP7 |
| PB8 | AP8 |
| PB9 | AP9 |
| PB10 | BBa_K2442101 |
| PBneg | dummy control |
Golden-gate-assembled mixtures were chemically transformed into DH5alpha cells for selection on Kanamycin plates. cPCR was carried out on seemingly-good colonies to screen for bands with correct size. Colonies with successful assemblies, indicated by cPCR, were liquid cultured for next-day miniprep. The retrieved plasmids, once verified through Sanger sequencing, were then transformed into a Marionette-Wild strain by electroporation. We chose Marionette as the testing strain because it is highly optimised as an arabinose sensor. Colonies of Marionette cells (selected on Kanamycin plates) were inoculated in 2 mL Neidhardt EZ Rich Defined Medium (EZRDM) and shaking-incubated overnight for 20 hours, at 37oC and 250 rpm.
We conducted two experiments to validate the nine pBADs against the wild-type counterpart and a negative control. The negative control (termed PBneg) was a dummy construct with similar length, containing mVenus CDS, but no araBAD promoters. Both the wild-type (PB10) and the PBneg constructs were cloned using the method described above.
Both experiments would involve measuring mVenus fluorescence intensity (FI) of transformed Marionettes grown under varying arabinose concentrations. FI was measured using a ClarioStar plate reader, on Greiner F-bottom 96-well plates or standard Greiner 384-well plates.
Results
Two-Level factorial screen
We first investigated the leakiness and maximal strength of the eight P_BADs. We chose arabinose concentrations of 0 uM and 1000 uM as the testing conditions. We shall, for now, not take Hill’s dynamics into account, and focus solely on the two concentration endpoints that best showcase the promoters’ two characteristics. We also calculated the fold-change (or fold-induction), defined as the ratio of maximal expression to leakiness:
Fold change = Max expression / Leakiness
Each sample was done in four replicates. The raw data are plotted in Figure 5,6 and 7.
Hill's induction assay
We also hoped to characterise classical parameters of inducible systems, namely, the dissociation constant KD and the Hill’s coefficient n. Therefore, we carried out a combinatorial induction assay with arabinose concentrations ranging from 0, 10, 25, 50, 75, 100, 125, 200, 500, 1000 and 2000 uM. For this testing, we used a 384-well plate instead to test all constructs; and a robot called Opentron OT-2 to automate plate preparation. Results are shown below.
We selected PB1, PB2, PB3, PB8, PB9 and PB10 and used the template equation given in Meyer et al (2019) for least-square regression. An example plot for the fitted curve of PB10 is given in Figure 10, and the six fitted functions are collected in a single plot in Figure 11.
| LVL1 ID | KD (uM) (fitted) | Hill's coefficient n (fitted) |
|---|---|---|
| PB1 | 29.6 | 0.54 |
| PB2 | 31.1 | 0.56 |
| PB3 | 32.9 | 0.54 |
| PB8 | 64.5 | 0.63 |
| PB9 | 140 | 0.67 |
| PB10 | 179 | 1.25 |
Discussion and outlook
Result from the 2LF-screen shows that PB1, PB2, PB3 and PB8 constructs (corresponding to promoter AP1, AP2, AP3 and AP8) showcased higher maximal expression but maintained same level of leakiness as that of PB10 (wild-type pBAD). AP2 and AP3 are definite contenders for significant improvements, being only half the length of their wild-type counterpart. We do not consider here AP8 as an improvement in our project, as this single rationale was fully accredited by the 2013 DTU Team. Still, it is worthwhile to note that in their previous characterization, the SPL (-35)-to-(-10) segment was incorporated into the wildtype length. Here, we put the optimised segment into the truncated 130 bp length, which also showed predictably high fold-change. We had slight issues with the induction assay. The experimental design failed to take into account the arabinose concentration range between 0 and 10uM. Therefore, for PB5, PB6, and PB7, we could not investigate the kinetics within this range. Our preliminary fitted curves for these functions (not shown) indicated a very steep, ‘all-or-nothing’ behaviour, and their maximal expression starts to plateau from even as low as 10uM. We hypothesised such observations with two possibilities:
- These promoters were ‘accidentally’ engineered to have a very sharp dynamic range (ie. Hill’s coefficient is very large) and thus confer a “switch-like” mechanism of action. In other words, only a minimal concentration of arabinose is enough to fully activate the promoter. (Note that this was not our original aim of improvement).
- These promoters still have predicted Hill’s kinetics, but the sigmoidal range (between 0 and 10uM) was not investigated thoroughly. This would mean that their dissociation constants are much smaller than that of the wild-type, shifting the functions left.
More careful testing must be done to verify these scenarios.
User Reviews
UNIQfdc01204f533dcdb-partinfo-00000000-QINU UNIQfdc01204f533dcdb-partinfo-00000001-QINU