Part:BBa_K4294755
36ntLuxI -sfGFP Transcriptional unit with the Ath5 Synthetic RBS (TetR Regulated)
The use of the TetR repressor system for the induction of the production of the luxI36nt-sfGFP gene (BBa_K4294207) controlled by our part Ath5 Synthetic RBS. To characterize different RBS variants in the luxI gene genetic context with fluorescent output.
Usage and Biology
This induction system is borrowed by the antibiotic resistance mechanism encountered in some bacteria species.Due to its structure aTc, can activate this system without having antibiotic action.[1]
Circuit Design
TetR forms a dimer and binds to tet operators in PTet promoter and blocks the assembly of the transcription machinery. PTet is a medium strength promoter that is constitutively “ON” in the absence of TetR. It contains two tet operators, one inside the core promoter sequence and the other after the -10 hexamer, leading to efficient repression. The system is efficiently induced via anhydrotetracycline hydrochloride (aTc), a tetracycline derivative which binds Tet R with an ~35-fold higher binding constant, being an effective inducer at very low concentrations. Additionally, its antibiotic activity is ~100-fold lower and has minimal toxic effect on E.coli cells in the concentration used for induction.The pTU1 high copy plasmid was used for this construct.
'Figure 1: Illustration of the 36nt LuxI- sfGFP fusion reporter protein.'
To prove the reliability and context independence of the BCDs, Mutalik et. al engineered several fusions of the first 36 nucleotides of commonly used regulators and enzymes with superfolder Green Fluorescent Protein (sfGFP). These first 36 nucleotides are sufficient enough to introduce a similar genetic context of the whole sequence they originated to the RBS [2]. sfGFP is an engineered version of the green fluorescent protein that can be fused with poorly folded peptides without misfolding and losing its functionality [3]., making these 36nt fusions a simple and probably reliable way to characterize translation rates provided by the same RBS for different coding sequences. A similar strategy was recently deployed to determine the effect of the spacer sequence between a RBS and the start codon in the context dependency of the RBS [4].
Based on the above information, we built a fusion of the first 36 nucleotides of the LuxI synthase with sfGFP to characterize the relative strength of our deployed RBS in the context of the LuxI coding sequence. sfGFP maintained its fluorescent properties and allowed us to conduct the characterisation
Measurment
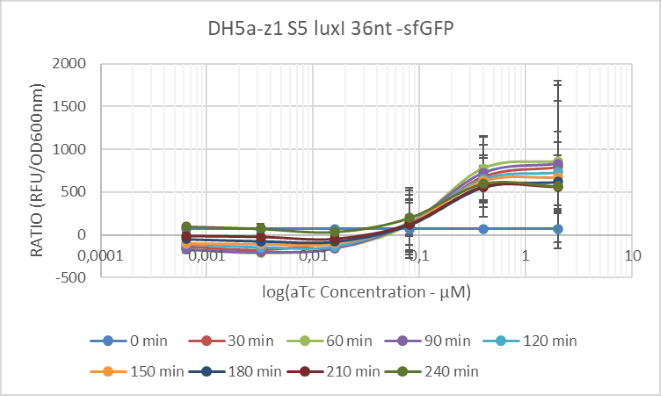
In our design the output was sfGFP fluorescent protein. To quantify this output we measured the fluorescence using microplate reader FlexStation3 (Molecular Devices) with excitation wavelength 485nm and emission wavelength 510nm derived from the fluorescent data base.We conducted measurements in different time points after the induction with aTc, using different concentrations of the inducer.
'Figure 2: Our results for S5 in different concentrations at different time points, the concentration range used for aTc was 2μM to 5,12 *10^(-6) performing 5-fold dilutions, the maximum induction time was 6 hours, here we present data until 4 hours, further induction did not result in increase of sfGFP production.'
Figure 3: Comparison of all 11 RBS as characterised by our measurements
References
[1] Bertram R, Hillen W. The application of Tet repressor in prokaryotic gene regulation and expression. Microb Biotechnol. 2008 Jan;1(1):2-16. doi: 10.1111/j.1751-7915.2007.00001.x. PMID: 21261817; PMCID: PMC3864427.
[2] Kudla G, Murray AW, Tollervey D, Plotkin JB. Coding-sequence determinants of gene expression in Escherichia coli. Science. 2009 Apr 10;324(5924):255-8. doi: 10.1126/science.1170160. PMID: 19359587; PMCID: PMC3902468.
[3] Pédelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol. 2006 Jan;24(1):79-88. doi: 10.1038/nbt1172. Epub 2005 Dec 20. Erratum in: Nat Biotechnol. 2006 Sep;24(9):1170. PMID: 16369541 [4]Duan Y, Zhang X, Zhai W, Zhang J, Zhang X, Xu G, Li H, Deng Z, Shi J, Xu Z. Deciphering the Rules of Ribosome Binding Site Differentiation in Context Dependence. ACS Synth Biol. 2022 Aug 19;11(8):2726-2740. doi: 10.1021/acssynbio.2c00139. Epub 2022 Jul 25. PMID: 35877551.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 559
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |