Part:BBa_K1330000
Spinach2.1 flanked by tRNALys3
This BioBrick contains the gene coding for the Spinach2.1 RNA, flanked by a tRNA scaffold sequence.
Usage and Biology
Spinach 2.1 RNA can bind and activate the fluorophore [http://pubs.acs.org/doi/abs/10.1021/ja410819x DFHBI-1T], both in vivo and in vitro. RNA molecules can thus be fluorescently tagged, by gene fusion with this BioBrick. The flanking tRNA comes from human tRNALys3 and increases stability and folding efficiency of the RNA, leading to higher fluorescent signal. It is possible to detect signal from Spinach2.1 when expressed from moderate strength promoters, e.g. the Anderson promoter library.
This part does not contain or need a Ribosome Binding Site.
Characterization
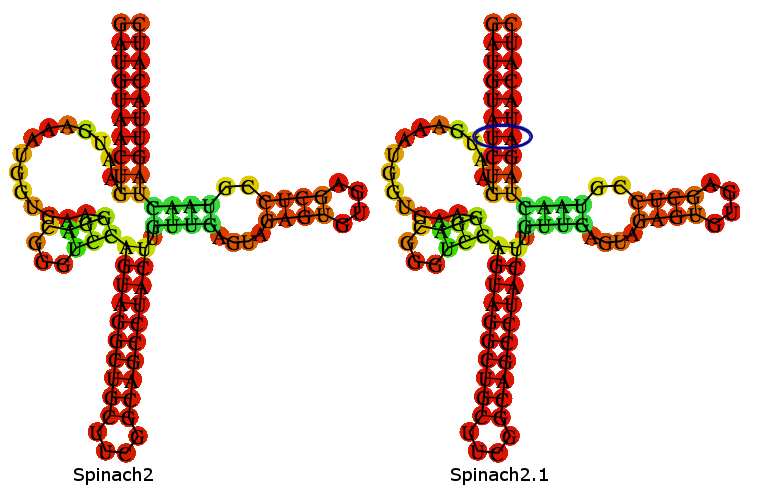
Spinach2.1 is derived from the Spinach2 sequence described in [http://www.nature.com/nmeth/journal/v10/n12/full/nmeth.2701.html Strack et al. (2013)]. Spinach2.1 has had 2 nucleotides swapped to remove a SpeI site, present in Spinach2.
A folding-prediction is shown below, demonstrating that Spinach2.1 is expected adopt the same fold as Spinach2.
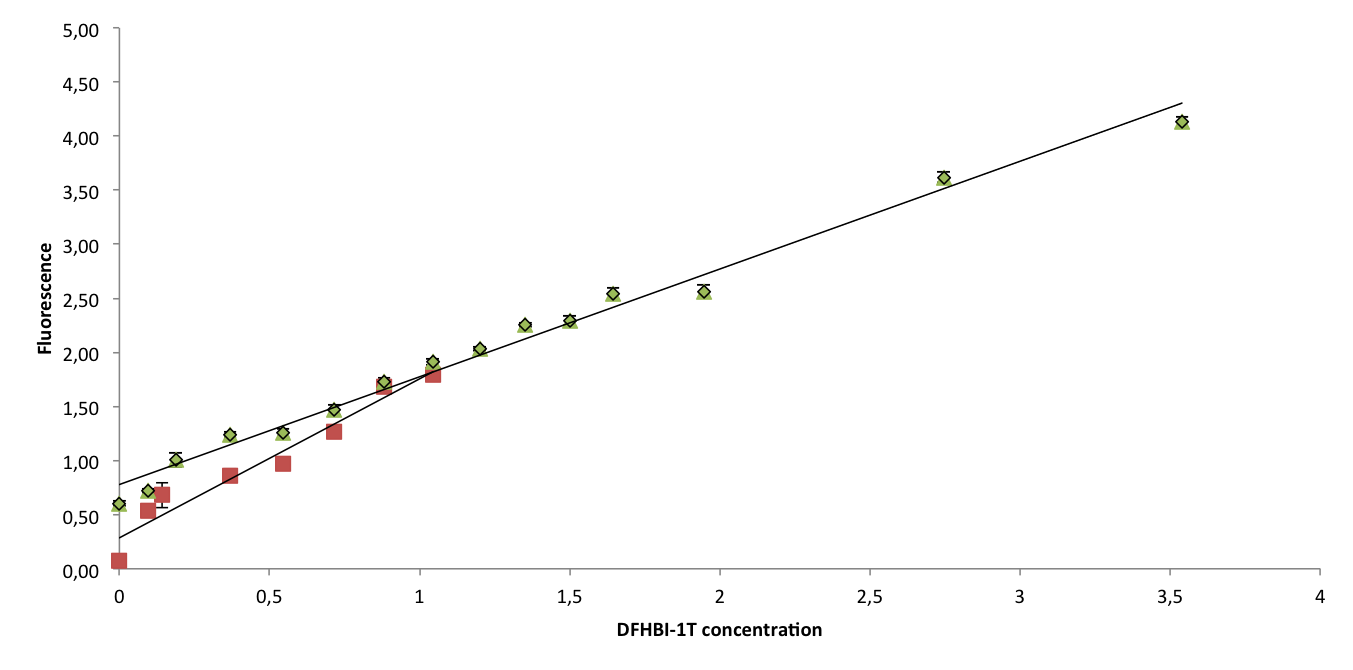
To confirm that Spinach2.1 functions as well as Spinach2, fluorescence measurements were conducted on Spinach2 and Spinach2.1 RNA in vitro. Fluorescence was measured on samples with different concentrations of DFHBI-1T and excess RNA, to compare the brightness of the Spinach-DFHBI-1T complexes. The resulting fluorescence standard curves are shown below:
The slopes of the curves represent the brightness of the RNA-DFHBI-1T complex. The slopes are similar, although Spinach2.1 might be moderately less bright. More experiments need to be conducted to establish whether the difference is significant.
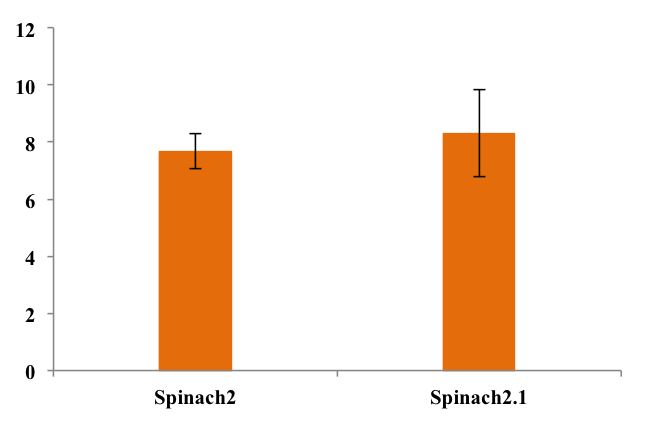
To assess folding efficiency of Spinach2.1 fluorescence was measured on samples with excess DFHBI-1T. Fluorescence normalized to RNA concentration is shown below:
As seen from the chart, there was no significant difference between fluorescence from the two RNA's. This means that equal fractions of RNA was bound to DFHBI-1T, indicating equal folding efficiency.
See [http://2014.igem.org/Team:DTU-Denmark/Achievements/Experimental_Results#lab-comparison-div our wiki] for more details.
2018 Team Hong_Kong-CUHK's Improvement
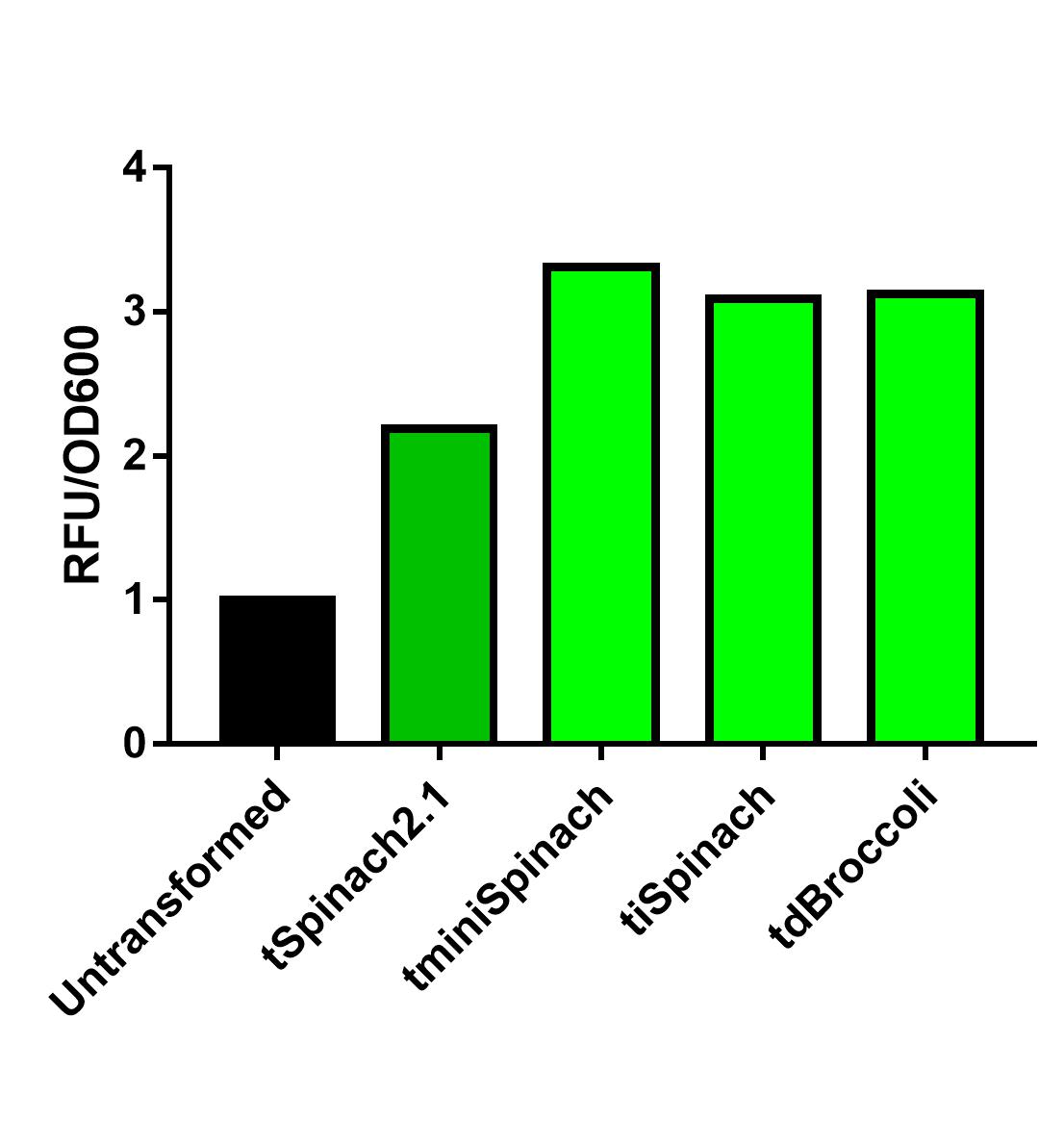
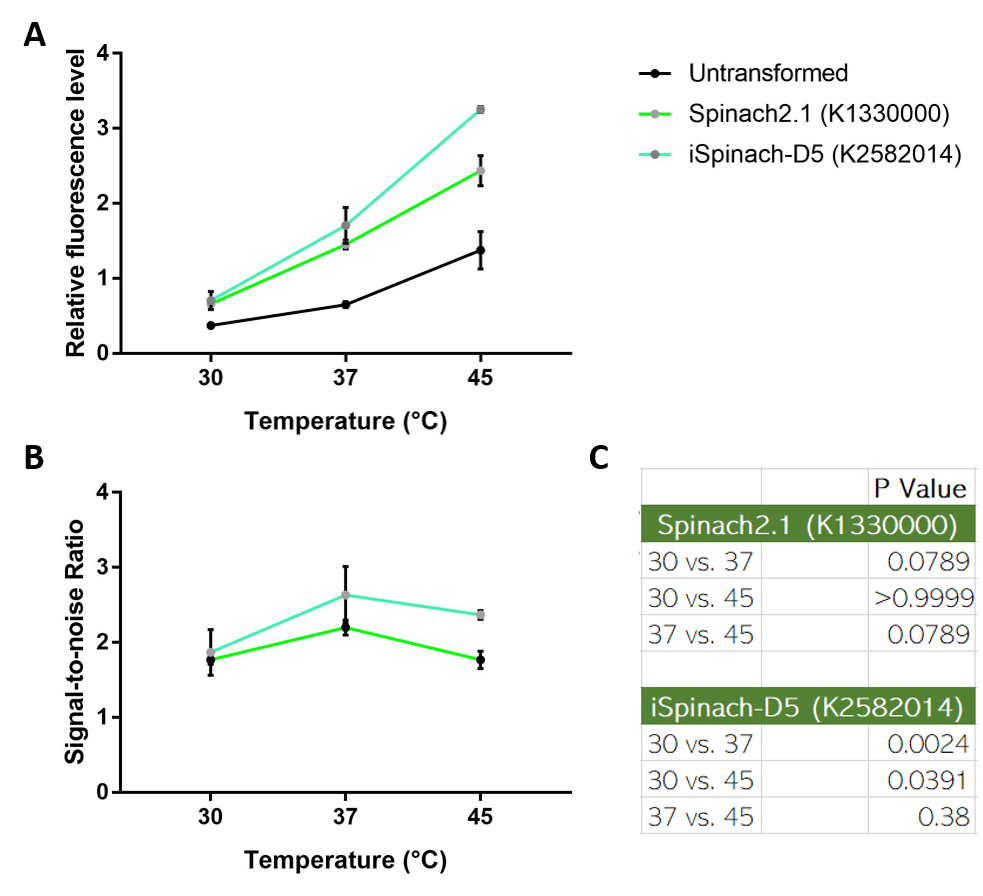
Spinach2.1 was originally constructed to prevent the illegal site in the superfolding Spinach2. However, its activity might be moderately less bright. On the other hand, Spinach2 has the melting temperature of ~38 degrees Celsius according to previous literature, which might not favor the measurement of heat-induced promoter activity. Comparing the 3 RNA reporters we constructed and the pre-existed Spinach2.1, iSpinach-D5 is moderately brighter.
Collaborating with NUS-A team, we also observed that iSpinach-D5 is moderately more resistant to 37->45 degrees Celsius change than Spinach2.1. However, it was also more vulnerable to 37->30 degrees Celsius change.
2022 Team IISER-Tirupati_India's Contribution
Contribution to BBa_K1330000:
Potential <a href="https://parts.igem.org/Part:BBa_K1330000">Spinach 2.1</a> mutants using Molecular Dynamic Simulations and MFE calculations with higher fluorescence intensity as compared to BBa_K1330000
Towards the modification of parts from the Registry of Standard Biological Parts (RSBP), we aimed to design investigations that would enhance the fluorescence intensity of Spinach2.1, a light-up aptamer [17]. In order to achieve this, we altered the sequence by introducing mutations (single, double), inversions, and deletions at different regions of the Spinach2.1 aptamer sequence to increase its relative fluorescence intensity than the wild-type. We employed two approaches in which, firstly, the sequence in the tetraloop (UUCG or TTCG) was mutated and inversion near this tetraloop sequence [10, 12]. Secondly, point mutations (A12G and U86C) were made at different places in the Spinach2.1 sequence. These mutations were chosen by a thorough inspection of the Spinach2.1 3D structure with the help of Dr. Hussain Bhukya, IISER Tirupati, and Dr. Harikrishna S, Senior Scientist at Syngene.
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/parts-mod-fig1.jpg' width="100%" height="auto" class = 'w3-center'>
Fig.1 Aptamer sequence and its RNAfold structure of Spinach2 and Spinach2.1 from the parts registry made by DTU, Denmark. The sequences highlighted in bold are the aptamer sequence, and those flanking are the tRNA scaffold sequence, tRNALys3. The sequences displayed are the DNA sequences of the RNA aptamers (Spinach2 and Spinach2.1) as given in the RSBP. The tetraloop region is indicated by a box colored blue.
All possible single-point mutations (SPMs) were made in the tetraloop using combinatorics and inversions. The free energy of the thermodynamic ensembles, frequency of the minimum free energy (MFE) structure, and the ensemble diversity were predicted using RNAfold. Based on these predictions, we chose a few sequences that displayed the best parameters for the above predictions. The mutated sequences and their corresponding RNAfold structures are given below.
Mutations and Inversion: Inversion near tetraloop and SPM in the loop:
Mutant 1 - Spinach2.2
The free energy of the thermodynamic ensemble for the sequence below is predicted to be -34.44 kcal/mol.
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/parts-mod-fig2.jpg' width="100%" height="auto" class = 'w3-center'>
Fig.2 Mutated aptamer sequence, Spinach2.2 and its RNAfold structure. The tetraloop is highlighted in red text and the inversions are underlined. The sequences highlighted in bold are the aptamer sequence and those flanking are the tRNA scaffold sequence, tRNALys3. The mutation and inversions are indicated in the RNAfold structures using blue circles.
Mutant 2 - Spinach2.3
The free energy of the thermodynamic ensemble for the sequence below is predicted to be -34.44 kcal/mol.
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/parts-mod-fig3.jpg' width="100%" height="auto" class = 'w3-center'>
Fig.3 Mutated aptamer sequence, Spinach2.3, and its RNAfold structure. The tetraloop is highlighted in red text, and the inversions are underlined. The sequences highlighted in bold are the aptamer sequence, and those flanking are the tRNA scaffold sequence, tRNALys3. The mutation and inversions are indicated in the RNAfold structures using blue circles.
Mutant 3 - Spinach2.4
The free energy of the thermodynamic ensemble for the sequence below is predicted to be -34.80 kcal/mol.
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/parts-mod-fig4.jpg' width="100%" height="auto" class = 'w3-center'>
Fig. 4 Mutated aptamer sequence, Spinach2.4 and its RNAfold structure. The tetraloop is highlighted in red text and the inversions are underlined. The sequences highlighted in bold are the aptamer sequence and those flanking are the tRNA scaffold sequence, tRNALys3. The mutation and inversions are indicated in the RNAfold structures using blue circles.
Mutant 4 - Spinach2.5
The free energy of the thermodynamic ensemble for the sequence below is predicted to be -35.50 kcal/mol.
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/parts-mod-fig5.jpg' width="100%" height="auto" class = 'w3-center'>
Fig. 5 Mutated aptamer sequence, Spinach2.5 and its RNAfold structure. The tetraloop is highlighted in red text and the inversions are underlined. The sequences highlighted in bold are the aptamer sequence and those flanking are the tRNA scaffold sequence, tRNALys3. The mutation and inversions are indicated in the RNAfold structures using blue circles.
B. In silico Analysis: Molecular Dynamics Simulation of RNA-ligand interactions to obtain energy
We modeled one PDB called 4TS2 [15] to show our idea is working. Because 4TS2 has maximum similarity with our desired part, Spinach 2.1 [17]. (90% match, verified through NCBI BLAST). We used Gromacs Umbrella Sampling MD [13] to obtain the free energy of the wild-type DFHBI-4TS2 complex. Later we replaced the 12th position A with G and 86th position U with C to generate a modified 4TS2 PDB file. We did the same Umbrella sampling for this mutated 4TS2 and compared the results between 4TS2 WT and 4TS2 Mutated.
The umbrella sampling method will tell the user about the binding energy between two species. The binding energy (ΔGbind) is obtained from the potential of mean force (PMF), extracted from a series of umbrella sampling simulations.
4TS2 Wild Type : [16]
ACGCGACCGAATGAAATGGTGAAGGACGGGTCCAGCCGGCTGCGCAGCCGGCTTGTTGAGTAGAGTGTGAGCTCCGTAACTGGTCGCGTC
GACGCGACCGAAUGAAAUGGUGAAGGACGGGUCCAGCCGGCUGCGCAGCCGGCUUGUUGAGUAGAGUGUGAGCUCCGUAACUGGUCGCGUC
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/wildtype.png' width="80%" height="auto" class = 'w3-center'>
4TS2 Mutated: [16]
GACGCGACCGAGTGAAATGGTGAAGGACGGGTCCAGCCGGCTGCGCAGCCGGCTTGTTGAGTAGAGTGTGAGCTCCGTAACCGGTCGCGTC
GACGCGACCGAGUGAAAUGGUGAAGGACGGGUCCAGCCGGCUGCGCAGCCGGCUUGUUGAGUAGAGUGUGAGCUCCGUAACCGGUCGCGUC
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/mutated.png' width="80%" height="auto" class = 'w3-center'>
TECHNIQUE FOR SIMULATION OF 4TS2 WILD TYPE OR MUTATED PDB IN GROMACS
We simulated the Spinach X-ray crystal structure (PDB entry: 4TS2) to validate the forcefield and other parameters for the MD as it was the closest (90% identity using NCBI BLAST) to our desired <a href="https://parts.igem.org/Part:BBa_K1330000">part</a> sequence. We employed Gromacs Umbrella Sampling MD to obtain the free energy of the wild-type DFHBI-4TS2 complex. Later, we mutated 12AG and U86C to generate the modified Spinach structure. The two structures (4TS2 and modified 4TS2) were then subjected to Umbrella Sampling using identical simulation parameters to compare the results.
Umbrella sampling method will tell the user about binding energy between two species. The binding energy (ΔGbind) is obtained from the potential of mean force (PMF), extracted from a series of umbrella sampling simulations.
RESULT: 4TS2 WILD TYPE PDB
At first, we did energy minimization and plotted it as the steepest descent algorithm, which converged in almost 200 steps. Then we did NVT equilibration and generated a Trajectory (XTC) and .gro file; from it, we can visualize how the simulation is occurring. We also plotted temperature Vs. Time and found fluctuations are significantly less, proving our equilibration is good enough. We did Steer MD Simulation (SMD) to remove the ligand from the pocket of G-Quadruplex with force 10000 KJ/mol/nm, and while visualizing its trajectory file in PyMol, we found that it is unbinding. We have also plotted SMD results as Spring force vs. time and get an umbrella-like graph. Then we generated configurations and put a window to start umbrella sampling using a bash script get_distances.sh.Further simulation will generatepotential of Mean Force Vs. Reaction Coordinate graph, from there we could conclude the binding energy.
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/energy-minimization-of-wild-4ts2.png' width="80%" height="auto" class = 'w3-center'>
Energy minimization for 4TS2-Wild type
( generated from potential.xvg file)
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/4ts2-wild-type-equillibration-graph.png' width="80%" height="auto" class = 'w3-center'>
Equilibration of 4TS2-Wild type
(Generated from temperature.xvg file)
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/4ts2-wild-type-smd.png' width="80%" height="auto" class = 'w3-center'>
Graph of SMD (Steered MD) of 4TS2-Wild type
(Generated from pullf.xvg)
RESULT: 4TS2 Mutated PDB
At first, we did Energy Minimization and plotted it as the steepest descent algorithm, and it converged in almost 177 steps. Then we did NVT equilibration and generated Trajectory(XTC) and .gro file; from it we can visualize how the simulation is occurring. We also plotted temperature Vs. Time and found fluctuations are significantly less, proving our equilibration is good enough. We did Steer MD Simulation(SMD) to remove the ligand from the pocket of G-Quadruplex with force 10000 KJ/mol/nm, and while visualizing its trajectory file in PyMol we found that it is unbinding. We have also plotted SMD results as Spring force vs. time and get an umbrella-like graph. Then we generated configurations and put a window to start umbrella sampling using a bash script get_distances.sh. Further simulation will generatepotential of Mean Force Vs. Reaction Coordinate graph, from there we could conclude the binding energy.
Below graphs are showing results for our simulation.
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/energy-minimization-of-mutated-4ts2.png' width="80%" height="auto" class = 'w3-center'>
Energy minimization for 4TS2-Mutated (generated from potential.xvg file)
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/4ts2-mutated-equillibration.png' width="80%" height="auto" class = 'w3-center'>
Equilibration of 4TS2-Mutated
(Generated from temperature.xvg file)
<img src = 'https://static.igem.wiki/teams/4438/wiki/contribution/modifications/4ts2-mutated-smd.png' width="80%" height="auto" class = 'w3-center'>
Graph of SMD (Steered MD) of 4TS2-Mutated
(Generated from pullf.xvg)
FINAL RESULT: However we couldn’t make it to PMF vs Reaction coordinate plot, energy minimization plot and other plots are indicative that 4TS2 mutated can have higher binding energy than the wild type’
The 4TS2 Wild type converges at 200 steps after energy minimization, while 4TS2 Mutated converges at 177 steps, hence it is more stable and predicted to give higher fluorescence.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
//classic/reporter
//dna/aptamer
| None |