Part:BBa_K1033001
4-coumarate ligase (4CL) with RBS
4-coumarate ligase (4CL) is an enzyme that catalyses the reaction from p-coumaric acid to 4-coumaroyl-coenzyme A (4-coumaryl-CoA). This enzyme is derived from the plant arabidhopsis thaliana, but exists in many other plants. [1]
In our project, we have been using it together with stilbene synthase, that produces resveratrol with the help of this enzyme.
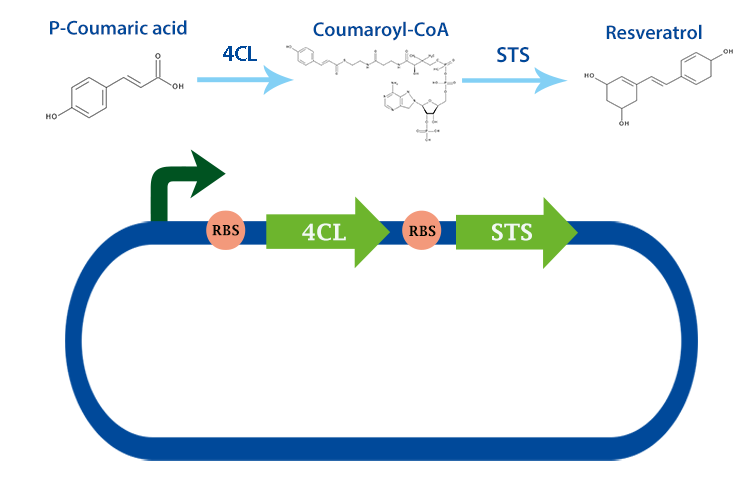
Applications With the help of this enzyme one can produce 4-coumaryl-CoA which is an important precursor in many metabolic pathways. For example, it can be used to produce resveratrol with stilbense synthase. [2]
KCL iGEM 2022
The 4-Coumarate:CoA Ligase is a member of the ANL superfamily of enzymes that includes luciferases and long-chain fatty acid CoA ligases. What unifies this superfamily is that they catalyze the adenylation of a carboxylate to form acyl-AMP followed by a second partial reaction that most often involves the formation of a thioester (Gulick, 2009).
Arabidopsis thaliana makes use of a 4CL enzyme for the synthesis of 4-coumaroyl-CoA, a precursor to a wide variety of compounds such as resveratrol or pterostilbene. 4CL is an anabolic enzyme and requires ATP to function. The stoichiometric formula is described below:
ATP + 4-coumarate + CoA = AMP + diphosphate + 4-coumaroyl-CoA
The enzyme At4CL works by accepting a carboxylate group compound and ATP in an adenylate-forming complex. Following hydrolysis of ATP, an acyladenylate intermediate is formed with the loss of two inorganic phosphates. This intermediate is then reacted with coenzyme A which forms a thioester bond, forming the many acyl sulfonamide analogues that form part of the Arabidopsis thaliana chemical repertoire (Watanebe et al., 2018).
One of these sulfonamide analogues is 4-coumaroyl-CoA. From this compound arises many pharmaceutically relevant compounds such as pterostilbene and resveratrol which have shown therapeutic potential in treating inflammatory diseases and neuroinflammation (Tomé-Carneiro et al., 2012). One noteworthy compound formed from a 4-coumaroyl-CoA precursor is the pancreatic amylase inhibitor Montbretin A which may have therapeutic potential in treating diabetes and obesity (Williams et al., 2015).
Common methods of investigating the enzymatic activity of At4CL involve a colourimetric assay. Colourimetric enzyme assays for 4-coumaroyl-CoA set colourimeters at 333 nm (Knolboch et al., 1975). Many assay kits are available online for determining enzymatic parameters of At4CL with many being designed for high throughput microplate protocols.
Subcellular Localisation
Determining the subcellular localisation of a protein using laboratory methods usually involves fluorescent protein labelling (e.g. with green fluorescent protein) coupled with fluorescence microscopy (Combs, 2010). However, these laboratory approaches have been superseded in precision by protein subcellular localisation prediction tools such as PSORTb and Proteome Analyst, and are more costly and laborious than the latter (Rey, Gardy, & Brinkman, 2005). Importantly, these computational approaches have limitations as a result of their high precision; for instance, a limitation of PSORTb is that the training datasets are limited for certain protein locations, which is mostly caused by its return of an “unknown” subcellular location if a confident prediction is not attained. Therefore, authors have emphasised the importance of complementing different computational prediction approaches to cover these pitfalls and reach a confident prediction (Gardy & Brinkman, 2006).
We took a general-to-specific approach when using different computational prediction tools to confidently characterise the At4CL mutant for its subcellular localisation, specifically regarding recombinant expression in bacteria. Two different tools, SignalP 5.0 (Armenteros et al., 2019) and PSORTb v3.0.3 (Yu et al., 2010) were used in this analysis. Firstly, SignalP detects potential signal peptides and cleavage sites across gram-negative and gram-positive bacteria, archaea, and eukarya. Then, PSORTb takes a multi-component approach whereby the amino acid sequence, motifs, signal peptides, and transmembrane helices are identified specific to 5 different bacterial locations. These were coupled to complement their distinct approaches in characterising At4CL for its subcellular localisation (Gardy & Brinkman, 2006).
Through SignalP, At4CL is predicted to not have any of the tested signal peptides from the many organism classes tested (Table 1). Further analysis for bacteria using PSORTb resulted in a confident prediction of At4CL localisation to the cytoplasm. Overall, the characterisation of the mutant At4CL for its predicted general subcellular localisation as well as for recombinant expression in microorganisms such as E. coli has provided a strong prediction of its localisation. Beyond characterisation itself, this also suggests that the expressed At4CL mutant is correctly located within the bacteria for pterostilbene production.
Table 1. SignalP Prediction for Probability of Signal Peptides in Different Organisms Values depict the probability (0 to 1) of the input amino acid sequence of the At4CL mutant to contain any of three types of signal peptides: Sec/SPI, Sec/SPII, and Tat/SPI, across Eukarya, Gram-positive and Gram-negative bacteria, and Archaea. Results are given to 3 decimal places.
Citations
Almagro Armenteros, J. J., Tsirigos, K. D., Sønderby, C. K., Petersen, T. N., Winther, O., Brunak, S., . . . Nielsen, H. (2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nature Biotechnology, 37(4), 420-423. doi:10.1038/s41587-019-0036-z
Gulick A. M. (2009). Conformational dynamics in the Acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS chemical biology, 4(10), 811–827. https://doi.org/10.1021/cb900156h
Tomé-Carneiro, J., Gonzálvez, M., Larrosa, M., Yáñez-Gascón, M. J., García-Almagro, F. J., Ruiz-Ros, J. A., García-Conesa, M. T., Tomás-Barberán, F. A., & Espín, J. C. (2012). One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. The American journal of cardiology, 110(3), 356–363. https://doi.org/10.1016/j.amjcard.2012.03.030
Williams, L. K., Zhang, X., Caner, S., Tysoe, C., Nguyen, N. T., Wicki, J., . . . Brayer, G. D. (2015). The amylase inhibitor montbretin A reveals a new glycosidase inhibition motif. Nature Chemical Biology, 11(9), 691-696. doi:10.1038/nchembio.1865
Knobloch, K.-H. et Hahlbrock, K.. (1975). Isoenzymes of p-Coumarate: CoA Ligase from Cell Suspension Cultures of Glycine max. European journal of biochemistry, 52(2), 311‑320. doi:10.1111/j.1432-1033.1975.tb03999.x
Watanabe, B., Kirikae, H., Koeduka, T., Takeuchi, Y., Asai, T., Naito, Y., . . . Hiratake, J. (2018). Synthesis and inhibitory activity of mechanism-based 4-coumaroyl-CoA ligase inhibitors. Bioorganic & Medicinal Chemistry, 26(9), 2466-2474. doi:https://doi.org/10.1016/j.bmc.2018.04.006
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 1108
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1675
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 1307
iGEM 2022 SHSBNU-China, new documentation (For Bronze)
Group: SHSBNU-China iGEM 2022
In this year’s iGEM competition, SHSBNU_China team focused on anthocyanins production, especially delphinidin. 4CL gene is one of the key genes in the synthetic pathway of delphinidin, which catalyzed phenylalanine into 4-coumaryl coenzyme A.
We synthesized the sequence and constructed into plasmid pETDuet.
After transforming to E.coli BL21 and induced by IPTG overnight at 16 degree Celsius at various concentrations (0.25 to 2 uM), we lysed the bacteria and performed SDS-PAGE and we confirmed 4CL enzyme to be expressed successfully. Judged by the thickness of the SDS-PAGE bandwidth and darkness, concentrations of iPTG don’t play a critical role in the expression of 4CL enzyme.
//collections/probiotics/production
| None |


