Part:BBa_K3717013
α-N-Acetylgalactosaminidase with T7 + RBS, C-Terminal 6x His-Tag, and Double Terminator
The composite part utilizes a T7 promoter + RBS (BBa_K525998), α-N-Acetylgalactosaminidase (BBa_K3717016), and double terminator (BBa_B0015).
α-N-Acetylgalactosaminidase catalyzes the cleavage of the terminal N-acetylgalactosamine of A type blood antigens such that the resultant antigen can be classified as an H antigen, which the anti-A and anti-B antibodies are unable to recognize and hence does not elicit an immune response in the human body [1]. Thus, α-N-Acetylgalactosaminidase can convert A blood types to universal O type.
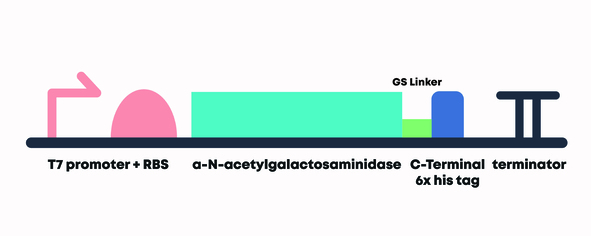
Figure 1. α-N-Acetylgalactosaminidase with T7 promoter, RBS and double terminator construct.
Construct Design
We derived the sequence of α-N-Acetylgalactosaminidase from Elizabethkingia meningoseptica [2] and optimized the sequence for E. coli protein expression. We then attached a 6x histidine tag (6x His-Tag) downstream of the α-N-Acetylgalactosaminidase sequence preceded by a glycine-serine linker (GS linker) to form our open reading frame (ORF) (BBa_K3717016) for purification purposes. We flanked our open reading frame with a T7 promoter + RBS (BBa_K525998) upstream of the open reading frame and a double terminator (BBa_B0015) downstream of the sequence. This composite part (BBa_K3717013) was assembled through DNA synthesis by IDT.
Characterization
Protein Expression and Purification
We transformed synthesized plasmids into BL21 (DE3) E. coli cells. We grew cultures at 37°C overnight, diluted those cultures, and then grew to OD600 0.5~0.6 at 37°C. We then induced expression with 0.5 mM IPTG and allowed cultures to grow overnight at room temperature. We harvested cells by centrifugation and lysed cell pellets through either sonication or with xTractor Lysis Buffer [3] supplemented with 20 mM imidazole. We purified our histidine-tagged proteins using Ni sepharose affinity chromatography. We then utilized SDS-PAGE to confirm the sizes of purified proteins.
Our results indicate a protein band at roughly 51.7 kDa, which is the molecular weight of our α-N-Acetylgalactosaminidase enzyme with the 6x His tag and GS linker attached, proving that our α-N-Acetylgalactosaminidase (Part: BBa_K3717013) was expressed and purified.

Figure 2. SDS-PAGE of purified proteins with the T7 promoter α-Galactosidase expressing construct (BBa_K3717009). Red triangles indicate expected size for the part.
Functional Testing
We confirmed the functionality of α-N-Acetylgalactosaminidase (NAGA) using two assays: colorimetric tests and mass spectroscopy.
Colorimetric Tests
We obtained a colorimetric substrate for NAGA from Sigma Aldrich (Fig 3). This substrate contains a 4-nitrophenol leaving group, which turns yellow upon successful cleavage in solution. The concentration of 4-nitrophenol was quantified at absorbance 405 nm using a 96-well plate-based assay [4].

Figure 3. Colorimetric substrates for NAGA.
We performed small-scale colorimetric tests to verify the function of our purified enzymes. To each well, we added 50 μL of 10 mM substrate, 10 μL of enzyme, 30 μL of water, and 10 μL of 10x Glycobuffer 1 (50 mM CaCl2, 500 mM sodium acetate, pH 5.5), a buffer recommended by New England Biolabs to ensure optimal enzyme activity [4]. Following 2 hours of reaction at room temperature, we diluted the well contents in 1.9 mL of water and took absorbance readings at 405 nm. Our results indicated that NAGA successfully cleaved the substrates (Fig 4). Moreover, we demonstrated the specificity of the enzyme as it did not cleave the substrate for other enzymes.

Figure 4. Small-scale colorimetrics tests verify the functionality and specificity of our purified NAGA. a) 96-well plates following enzyme reaction. b) absorbance readings of diluted well contents. c) buffers and solutions used in substrate and control wells.
To quantify the activity of NAGA, we performed enzyme reactions at various substrate concentrations and measured the absorbance at different time intervals over a constant time period (Fig 5). We used the averages from at least three independent trials to calculate the Michaelis–Menten constant for enzyme efficiency (see enzyme model in modeling). The results of our colorimetric substrate tests show that NAGA enzyme is specific and functions as expected.

Figure 5. Absorbance over time for various NAGA substrate concentrations.
Mass Spectroscopy Trisaccharide Antigen Tests
Given that the colorimetric results were positive, we wanted to further test the enzyme’s specificity by using trisaccharides as substrates. These trisaccharides contain three monosaccharides that have identical chemical structures to the A and B antigens found on RBCs. To test a proof of concept functionality of NAGA in cleaving the A-antigen trisaccharide, we carried out a reaction of the enzyme and its trisaccharide dissolved in 1X GlycoBuffer [5], deionized water, and purified BSA for 1 hour at 37°C. The A-antigen trisaccharides were kindly supplied by Professor Dr. Todd Lowary from the Institute of Biological Chemistry at Academic Sinica [6].
For the enzyme tests, a non-specific trisaccharide served as a negative control and A-antigen trisaccharide specific to NAGA served as the experimental unit (Table 1).
Table 1. Experimental setup for enzyme-antigen tests.

After the reaction, the reaction solution was passed through C18 columns to minimize impurities from the reaction solution. We then evaluated the flow through solution with a mass spectrometer to measure peaks in molar mass in order to determine the compounds present in the reaction solution. The original molar mass of the A-antigen is 713.35 g/mol; therefore, after cleavage, we expected two fragments with molar masses of 510.27 g/mol and 203.08 g/mol to form. (Fig 6).

Figure 6. Molecular structure and mass of A-antigen trisaccharides and subsequent products following enzymatic cleavage.
The results shown by the mass spectrometer confirm the functionality of NAGA. The experimental unit shows the presence of the cleaved fragment from the reaction with A-antigen trisaccharide, indicated by the peak at 533 g/mol in the mass spectrum (Fig 7). The negative control confirms the specificity of NAGA, since it did not cleave the B-antigen trisaccharide, as indicated by the peak at 695 g/mol (Fig 8). Throughout all readings of the mass spectrum, the molar mass of the peaks have been increased by 23 g/mol due to a sodium adduct. Unrelated peaks/background noise can be attributed to compounds present in the buffer solution.

Figure 7. α-N-Acetylgalactosaminidase cleaves A-antigen trisaccharide.Mass spectrum of α-N-Acetylgalactosaminidase and A-antigen trisaccharide reaction solution (experimental unit, flow through). The peak at 533g/mol shows the presence of the cleaved fragment from the reaction of the α-N-Acetylgalactosaminidase enzyme and A-antigen trisaccharide.

Figure 8. Negative Control: Mass spectrum of α-N-Acetylgalactosaminidase and B-antigen trisaccharide reaction solution (negative control, flow through). The negative control confirms the specificity of α-N-Acetylgalactosaminidase, as the B-antigen trisaccharide was not cleaved, indicated by the peak at 695g/mol
Mass spectroscopy results from the reaction between NAGA and A-antigen trisaccharide substrate demonstrate its ability to cleave its specific trisaccharides, demonstrating the proof of concept that the enzyme is able to cleave the A blood group antigens.
References
1. Rahfeld, Peter, and Stephen G. Withers. “Toward Universal Donor Blood: Enzymatic Conversion of A and B to O Type.” Journal of Biological Chemistry, vol. 295, no. 2, Jan. 2020, pp. 325–34. DOI.org (Crossref), https://doi.org/10.1074/jbc.REV119.008164.
2. UniProtKB - A4Q8F7 (GH109_ELIME). UniProt, 2 June 2021, www.uniprot.org/uniprot/A4Q8F7. Accessed 20 Oct. 2021.
3. XTractorTM Buffer & xTractor Buffer Kit User Manual. (n.d.). 10.
4. Held, Paul. “Kinetic Analysis of β-Galactosidase Activity Using the PowerWave™ HT and Gen5™ Data Analysis Software .” BioTek, 16 Feb. 2007.
5. Biolabs, New England. “Typical Reaction Conditions for α-N-Acetylgalactosaminidase (P0734).” New England Biolabs: Reagents for the Life Sciences Industry, https://international.neb.com/protocols/2013/01/10/typical-reaction-conditions-p0734.
6. Meloncelli, Peter J., and Todd L. Lowary. “Synthesis of Abo Histo-Blood Group Type I and II Antigens.” Carbohydrate Research, vol. 345, no. 16, 16 Sept. 2010, pp. 2305–2322., https://doi.org/10.1016/j.carres.2010.08.012.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 567
- 1000COMPATIBLE WITH RFC[1000]
| None |
