Part:BBa_K3806016
Theophylline-binding aptazyme regulating lacZ expression (semi-cRBS). With T7 promoter.
prov
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 3204
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 2321
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Sensing small molecules is the foundation of many applications, ranging from disease diagnosis, prognosis, or treatment to detecting small pollutants in the environment. Synthetic genetic switches are a promising tool to detect and quantify small molecules. Conventional synthetic biology-based biosensors are based on transcription factors. However, transcription factors are not easily reprogrammable with respect to ligand selectivity. Antibody-based biosensors can be easily developed into new sensing capabilities. Unfortunately, these types of sensors are not suitable to detect low molecular weight compounds. RNA-based biosensors are an interesting alternative since they present a remarkable flexibility to be engineered into sensing a wide range of analytes with high sensitivity and selectivity. In particular, aptazymes, ligand regulated self-cleaving ribozymes, are of special interest as cleavage of the aptazyme can be coupled to regulated gene expression in vivo or in vitro. Moreover, newly developed methods such as DRIVER (de novo rapid in vitro evolution of RNA biosensors) enable rapid, automated, and multiplexed engineering of aptazymes sequences to diverse ligands.
The TU Delft 2021 team provides the iGEM community with a novel genetic switch construct (BBa_K3806016) in which an aptazyme sequence is fused to the lacZ reporter gene to convert a ligand concentration to a colorimetric read-out. The BBa_K3806016 contains a theophylline-binding aptazyme sequence [1], yet this part is designed to be modular and serve as a template to engineer other ligand-specific genetic circuits by swapping the aptazyme domain. This part was successfully expressed in a cell-free system, and its biosensing performance was compared to BBa_K3806014, showing an improvement in the dynamic range and time-response capabilities.
Aptazyme-regulated gene expression mechanism
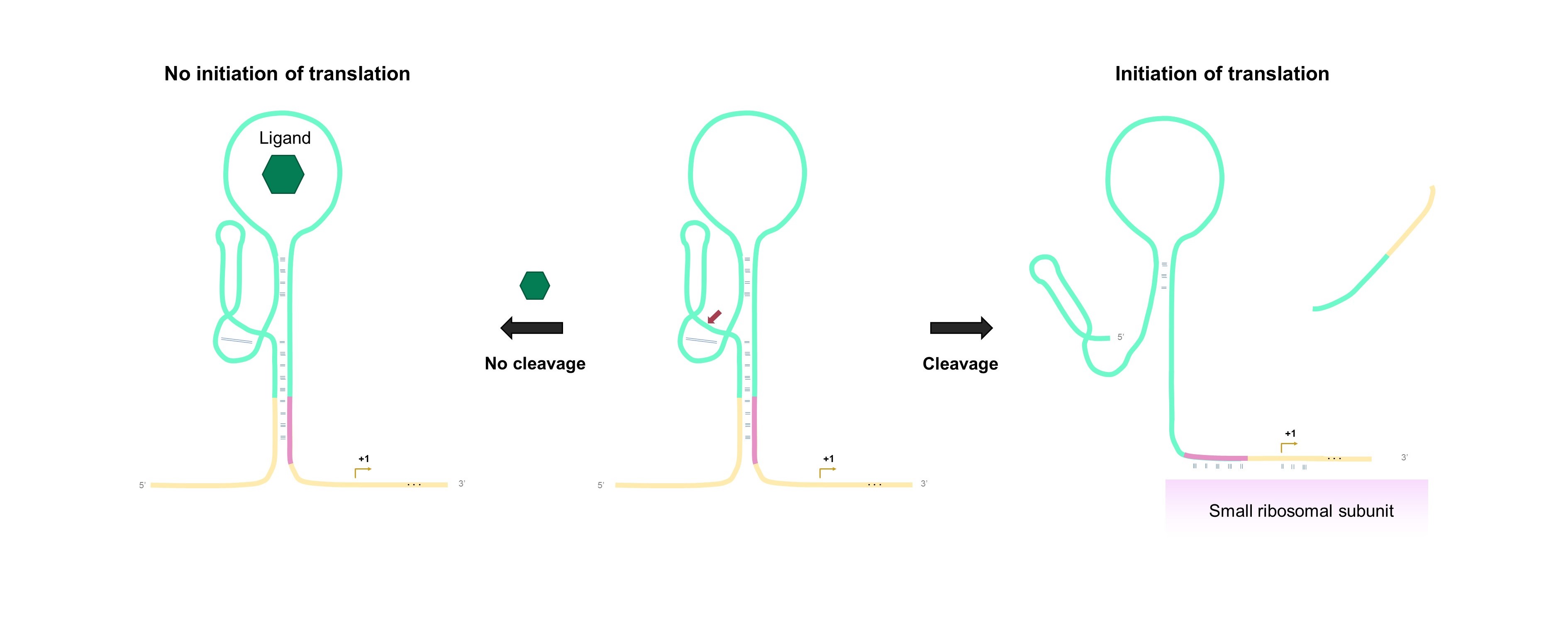
The aptazyme-regulated expression of lacZ depends on the accessibility of the ribosomal binding site (RBS) for translation initiation, inspired by the study of Klauser & Hartig [2]. After transcription of the DNA template comprising the aptazyme and fused lacZ reporter gene, a part of the RBS is hidden in the stem of the aptazyme. When the aptazyme is stabilized upon binding of the ligand, the RBS remains partly sequestered by its antisense strand, and translation is mostly prohibited. Cleavage of the aptazyme in the absence of the ligand liberates the RBS (Fig. 1). As a result, the ribosome can bind to the RBS and translate the downstream reporter gene to β-galactosidase protein. Subsequently, β-galactosidase can catalyze the colorimetric conversion of the enzymatic substrates such as chlorophenol red-b-D-galactopyranoside (CPRG) and X-gal.
Fig. 1 Aptazyme-regulated gene expression mechanism. Binding of the ligand renders a catalytically inactive aptazyme, the RBS remains partly sequestered by its antisense strand, repressing translation (left). In the absence of the ligand, self-cleavage of the aptazyme frees the RBS, resulting in the binding of the small ribosomal subunit and initiation of translation (right).
| None |