Part:BBa_K1365006
NisR and NisK
Usage and Biology
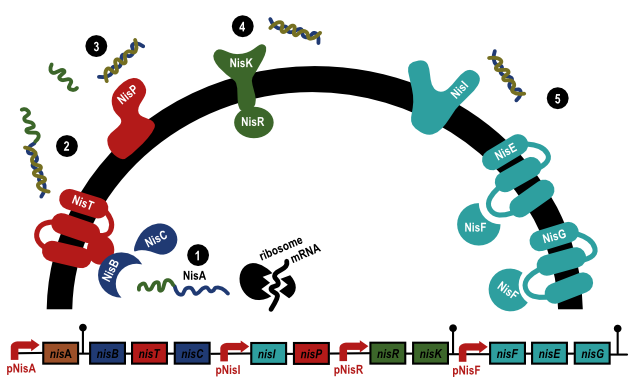
This BioBrick contains the coding parts for the genes NisR and NisK. NisK is a membrane-associated protein kinase that phosphorylates NisR in response to nisin in the environment. NisR is a regulatory protein, regulating promoters like PNisA and PNisI.
Together with the genes NisA, NisB, NisC, NisP they are responsible for producing the lantibiotic nisin in Lactococcus lactis, see the figure below. The NisA protein is first modified and then transported out of the cell. The serines and threonines of NisA are dehydrated by NisB and then the precursor is cyclized by NisC. After this process, the precursor is transported out of the cell (2). Here, the lead peptide is cut off by NisP (3) and the mature nisin is formed.1
Nisin is a lantibiotic, an bacteriocidal peptide. Nisin inhibits the growth of a broad range of Gram positive bacteria, of which many are spoilage bacteria or pathogens. Nisin is therefore extensively used in the food industry as a preservative. Nisin forms pores in the membrane of the bacteria it kills and inhibits the peptidoglycan synthesis.2
Sequence and features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
1. Cheigh, C. I. and Pyun, Y.R. (2005) Nisin biosynthesis and its properties. Biotechnol. Lett. 27: 1641-1648
2. Zhou, H. et al. (2014) Mechanisms of nisin resistance in Gram-positive bacteria. Ann. Microbiol. 64: 413-420
Contribution by Team IISER_Kolkata 2021
Group: iGEM21_IISER_Kolkata
Author: Mahapatra Anshuman Jaysingh, Shubhamay Das
Summary: This year our team focuses on one segment of this part which is nisA. NisA gene is responsible for the production of the 57-amino acid long pre-peptide. This 57-amino acid comprises a leader peptide, cleavage of which produces a 34 amino acid long Nisin A protein. Nisin Resistance Protein (NSR) is a protein that is expressed on the cell surface of certain pathogenic strains like Streptococcus agalactiae, Streptococcus uberis. NSR is responsible for inactivating nisin by proteolytically cleaving the peptide bond between MeLan28 and Ser29 resulting in a truncated nisin (nisin1–28). NisinPV is a synthetically designed mutant of NisinA with the Mutation of Serine to proline at position 29 (S29P) combined with isoleucine to valine at position 30 (I30V). Nisin PV (BBa_K379904) shows enhanced antimicrobial activity against Streptococcus uberis by inhibiting biofilm formation and decreasing its viability. In the section below we have shown a comparative analysis of the stability of NisinA and NisinPV under the interaction of Nisin Resistence protein.
Interaction with Nisin Resistance Protein:The data in this section is adapted from the simulations by Field, Des et al. They carried out MD simulations to predict the difference in molecular dynamics of the NSR protein in its interaction with the nisin C-terminus (residues 22–34) for nisin-A and nisin PV models.
Molecular docking:We tried to replicate the setup used by Des et al. For the NSR (Nisin Resistance protein) molecule, the PDB file 4Y68 was used and for the Nisin the 1WCO PDB file was used. NisinA structure was mutated using PyMol and a PRO-VAL mutation was added to the 29th and 30th residue respectively. To determine a starting configuration for the NSR-nisin complex a docking program Autodock for ligand and protein binding was used where the Nisin (A/PV) was set as ligand and NSR was set as the receptor. We first performed the docking of NisinA with the tunnel region of NSR and selected the lowest energy stage as the starting configuration. The docs location was observed to be consistent with the ligand-binding site of Nisin as discussed in the literature. The ligand-binding site for NisinA was noted and NisinPV was docked using the same coordinates.
| None |