Ribosome Binding Sites/Prokaryotic/Constitutive/Anderson
Description
The Anderson RBS family are suitable for general protein expression in E. coli or other prokaryotes. The family is known to cover a range of translation initiation rates so by testing a few family members it should be possible to find a translation initiation rate that suits your application. The family of RBS were recovered from a library screen by Chris Anderson. In the original library, 6 nucleotide locations close to where the ribosome binds were randomized (see the master sequence below). The family members have not yet been quantitatively characterized (see the Characterization section below).
Obtaining Anderson RBS parts
The sequences of the Anderson RBS parts can be found in the table below. To obtain the physical DNA, we recommend two approaches -
Via de novo synthesis: Since the RBS parts are short sequences, they can be easily and cheaply ordered as two single-stranded complementary oligo's and annealed. See here for a tutorial on how to construct short parts via oligo annealing.
Via the Registry distribution: The RBS parts are included in the Registry distribution. These parts are cloned in plasmid pSB1A2, but there is also a constitutive promoter (derived from BBa_J23100) inserted into the XbaI site. So, for example, the EcoRI/PstI region of part BBa_J61100 reads:
| ...Biobrick Prefix..................J23100.................XbaI....RBS Part.....Biobrick Suffix... |
| gaattcgcggccgcttctagaGTTGACGGCTAGCTCAGTCCTAGGTACAGTGCTAGCTtctagaGAAAGAGGGGACAAactagtagcggccgctgcag |
This feature in no way prevents the use of these parts in standard Biobrick assembly. Normal prefix insertion into EcoRI/XbaI will delete this promoter element. Suffix insertion into SpeI/PstI will retain this promoter, but it can of course be removed later by a prefix insertion. Note also that the T at the start of the BioBrick suffix was randomized during library construction and is therefore rarely a T. Because the Registry does not permit variation at this position, the Registry sequence of composite parts derived from these RBS will not match the physical sequence
Anderson RBS family members
| Identifier | Sequencea | Predicted Strengthb | |
|---|---|---|---|
| Mean | CV | ||
| Master Sequence | TCTAGAGAAAGANNNGANNNACTAGATG | ||
| BBa_J61100 | TCTAGAGAAAGAGGGGACAAACTAGATG | 1 (12531) | 1.32 |
| BBa_J61101 | TCTAGAGAAAGACAGGACCCACTAGATG | 0.41 (5180) | 1.15 |
| BBa_J61102 | TCTAGAGAAAGATCCGATGTACTAGATG | 0.12 (1548) | 1.10 |
| BBa_J61103 | TCTAGAGAAAGATTAGACAAACTAGATG | 0.08 (1061) | 1.21 |
| BBa_J61104 | TCTAGAGAAAGAAGGGACAGACTAGATG | 0.77 (9651) | 1.20 |
| BBa_J61105 | TCTAGAGAAAGACATGACGTACTAGATG | 0.10 (1295) | 1.07 |
| BBa_J61106 | TCTAGAGAAAGATAGGAGACACTAGATG | 1.75 (21887) | 1.16 |
| BBa_J61107 | TCTAGAGAAAGAAGAGACTCACTAGATG | 0.22 (2753) | 0.92 |
| BBa_J61108 | TCTAGAGAAAGACGAGATATACTAGATG | 0.34 (4270) | 1.01 |
| BBa_J61109 | TCTAGAGAAAGACTGGAGACACTAGATG | 0.75 (9345) | 1.32 |
| BBa_J61110 | TCTAGAGAAAGAGGCGAATTACTAGATG | 0.58 (7254) | 1.07 |
| BBa_J61111 | TCTAGAGAAAGAGGCGATACACTAGATG | 0.55 (6945) | 1.13 |
| BBa_J61112 | TCTAGAGAAAGAGGTGACATACTAGATG | 0.57 (7130) | 1.49 |
| BBa_J61113 | TCTAGAGAAAGAGTGGAAAAACTAGATG | 0.20 (2484) | 1.59 |
| BBa_J61114 | TCTAGAGAAAGATGAGAAGAACTAGATG | 0.32 (3996) | 0.98 |
| BBa_J61115 | TCTAGAGAAAGAAGGGATACACTAGATG | 0.78 (9796) | 1.22 |
| BBa_J61116 | TCTAGAGAAAGACATGAGGCACTAGATG | 1.31 (16357) | 1.09 |
| BBa_J61117 | TCTAGAGAAAGACATGAGTTACTAGATG | 0.20 (2549) | 1.25 |
| BBa_J61118 | TCTAGAGAAAGAGACGAATCACTAGATG | 0.17 (2117) | 1.12 |
| BBa_J61119 | TCTAGAGAAAGATTTGATATACTAGATG | 0.08 (1002) | 1.20 |
| BBa_J61120 | TCTAGAGAAAGACGCGAGAAACTAGATG | 0.29 (3670) | 1.47 |
| BBa_J61121 | TCTAGAGAAAGAGACGAGTCACTAGATG | 0.23 (2879) | 1.26 |
| BBa_J61122 | TCTAGAGAAAGAGAGGAGCCACTAGATG | 1.87 (23410) | 1.23 |
| BBa_J61123 | TCTAGAGAAAGAGATGACTAACTAGATG | 0.12 (1495) | 0.94 |
| BBa_J61124 | TCTAGAGAAAGAGCCGACATACTAGATG | 0.13 (1624) | 1.20 |
| BBa_J61125 | TCTAGAGAAAGAGCCGAGTTACTAGATG | 0.17 (2123) | 1.28 |
| BBa_J61126 | TCTAGAGAAAGAGGTGACTCACTAGATG | 0.34 (4273) | 1.24 |
| BBa_J61127 | TCTAGAGAAAGAGTGGAACTACTAGATG | 0.19 (2390) | 1.75 |
| BBa_J61128 | TCTAGAGAAAGATAGGACTCACTAGATG | 0.28 (3479) | 1.16 |
| BBa_J61129 | TCTAGAGAAAGATTGGACGTACTAGATG | 0.38 (4736) | 1.24 |
| BBa_J61130 | TCTAGAGAAAGAAACGACATACTAGATG | 0.09 (1181) | 1.09 |
| BBa_J61131 | TCTAGAGAAAGAACCGAATTACTAGATG | 0.12 (1466) | 1.02 |
| BBa_J61132 | TCTAGAGAAAGACAGGATTAACTAGATG | 0.48 (5984) | 1.15 |
| BBa_J61133 | TCTAGAGAAAGACCCGAGACACTAGATG | 0.49 (6084) | 0.86 |
| BBa_J61134 | TCTAGAGAAAGACCGGAAATACTAGATG | 0.49 (6099) | 1.05 |
| BBa_J61135 | TCTAGAGAAAGACCGGAGACACTAGATG | 1.68 (21059) | 1.07 |
| BBa_J61136 | TCTAGAGAAAGAGCTGAGCAACTAGATG | 0.14 (1755) | 1.38 |
| BBa_J61137 | TCTAGAGAAAGAGTAGATCAACTAGATG | 0.06 (787) | 1.11 |
| BBa_J61138 | TCTAGAGAAAGATATGAATAACTAGATG | 0.09 (1118) | 1.07 |
| BBa_J61139 | TCTAGAGAAAGATTAGAGTCACTAGATG | 0.22 (2754) | 1.25 |
aThe sequence of individual RBS are shown in black and red. The grey nucleotides show the bracketing sequence that results from assembling the RBS with an upstream part and a downstream coding sequence. The start codon of the downstream coding sequence is shown in green. See the "Obtaining Anderson RBS parts" section above for a description of how the physical DNA sequence of the Anderson RBS parts in the Registry differs slightly from the BioBrick® standard. cThe predicted relative strengths of these RBS parts were calculated by Howard Salis. The first number is the mean RBS strength normalized by the strength of No part name specified with partinfo tag.. The second number (in parentheses) is the mean RBS strength prior to normalization to facilitate comparison with RBS parts from other collections. CV is an abbreviation for Coefficient of Variation (Standard deviation/Mean). A description of the method for predicting RBS part strength is provided here.
Characterization
Measured strengths
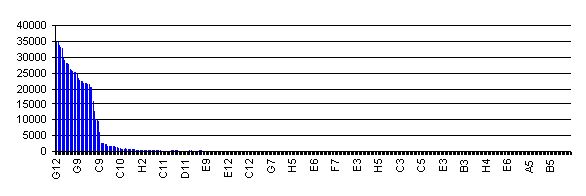 Relative RFP levels from reporters using different clones from the RBS library (Click the chart for a larger version). |
| Raw data for the library after sequencing |
| Plate Reader data of the clones before repooling / FokI fragmentation |
To date, the RBS library has not been experimentally characterized. This is largely a reflection of the method used to construct the library. Initiatially, all members of the library had the RBS variant upstream of an RFP gene. Red fluorescence for 384 individual library members, and the ranked activity of them is shown in the chart (right). All screened clones showing activity detectable above background were combined into 7 activity pools. Each pool underwent a procedure designed to remove the RFP gene and put back the SpeI site in each part. 96 individual processed clones were then sequenced, and the family of RBS parts reflects all the unique clones.
So, at this stage we really can't say much about their relative activity. All I can tell you is that they should all initiate translation at a rate above background, and they are very roughly in decreasing order of activity. RBS's with low numbers are likely to be stronger RBS's than those with higher numbers.
...and of course YOU could do some characterization and post the info here.
Predicted Strengths
135 RBS parts and 144 protein coding sequences from the Registry were analyzed. The sequence of each RBS part was placed in front of each coding sequence. The relative expression level of this RNA sequence was predicted using Howard Salis' RBS calculator (v1.0). At the same time, the likelihood that the RNA sequence is at equilibrium is also estimated. The predictions of the RBS calculator are most accurate when the RNA sequence is at or near equilibrium. Then, the mean translational activity of an RBS part over all protein coding sequences is calculated. This mean only includes the sequences that are estimated to be at equilibrium. In the table above the mean strengths of the RBS parts is reported relative to BBa_B0034. The coefficient of variation (CV, the ratio of the standard deviation to the mean across all coding regions tested) gives a measure of how likely the strength of the RBS is to vary depending on the sequence context in which the RBS is used.
Note: The original nomenclature was Bca1050 and then Bca9110.

