Part:BBa_K3552002
PaPilA
PaPilA is a type 4 pilus generally on Pseudomonas aeruginosa which can conduct electricity. This part is in the part collection where we provide different conductive pilus based on the same pilin generator. By these different type 4 pilus we can compare the qualities.
The part collection includes: Parts that are different kinds of type 4 pilus: BBa_K3552000 BBa_K3552001 BBa_K3552002. Parts that are the generator of the type 4 pilus: BBa_K3552003 BBa_K3552004 BBa_K3552005 BBa_K3552006 BBa_K3552007 BBa_K3552008 BBa_K3552018 BBa_K3552019 BBa_K3552020 BBa_K3552021 BBa_K3552022 BBa_K3552023 BBa_K3552024 BBa_K3552025 BBa_K3552026 BBa_K3552027 BBa_K3552028 BBa_K3552029. Parts that are a complete circuit: BBa_K3552009 BBa_K3552010 BBa_K3552011 BBa_K3552012.
Our part collection can instruct other teams to designed new rechargeable pilus and substitution of different major pilin.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Reference
Liu, Xi et al. “Biological synthesis of high-conductive pili in aerobic bacterium Pseudomonas aeruginosa.” Applied microbiology and biotechnology vol. 103,3 (2019): 1535-1544. doi:10.1007/s00253-018-9484-5
Usage and Biology
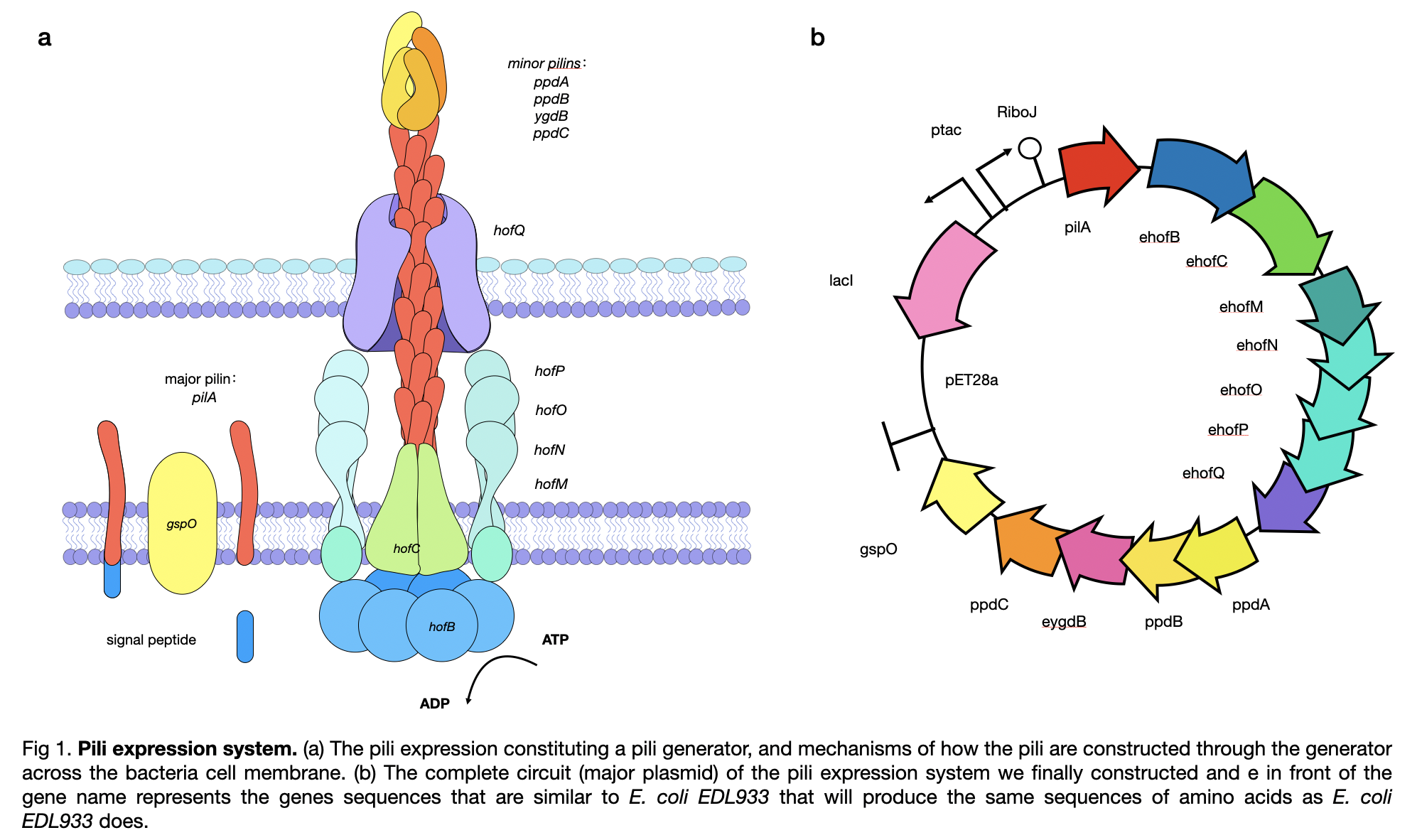
PaPilA are fimbrial protein which normally grow on Pseudomonas aeruginosa, but our PapilA is an artificially modified gene to enable it to conduct electricity. They are type 4 pili which are long and thin that displayed on the cell surface membrane. They can promote adherence, motility and transport functions in the bacteria. They are mainly built as helical polymers of a single subunit called the major pilin. These pilins are initially in the plasma membrane and the N-terminal is positively charged. The mature pilins will be extracted through the membrane by the pilin generator. The naturally occurred PaPilA contains ß-sheet and ∂ß-loop on the C-terminal which prevents them from conducting electricity at the beginning. This part is removed in our design to maintain same structure as GsPilA.
Characterization
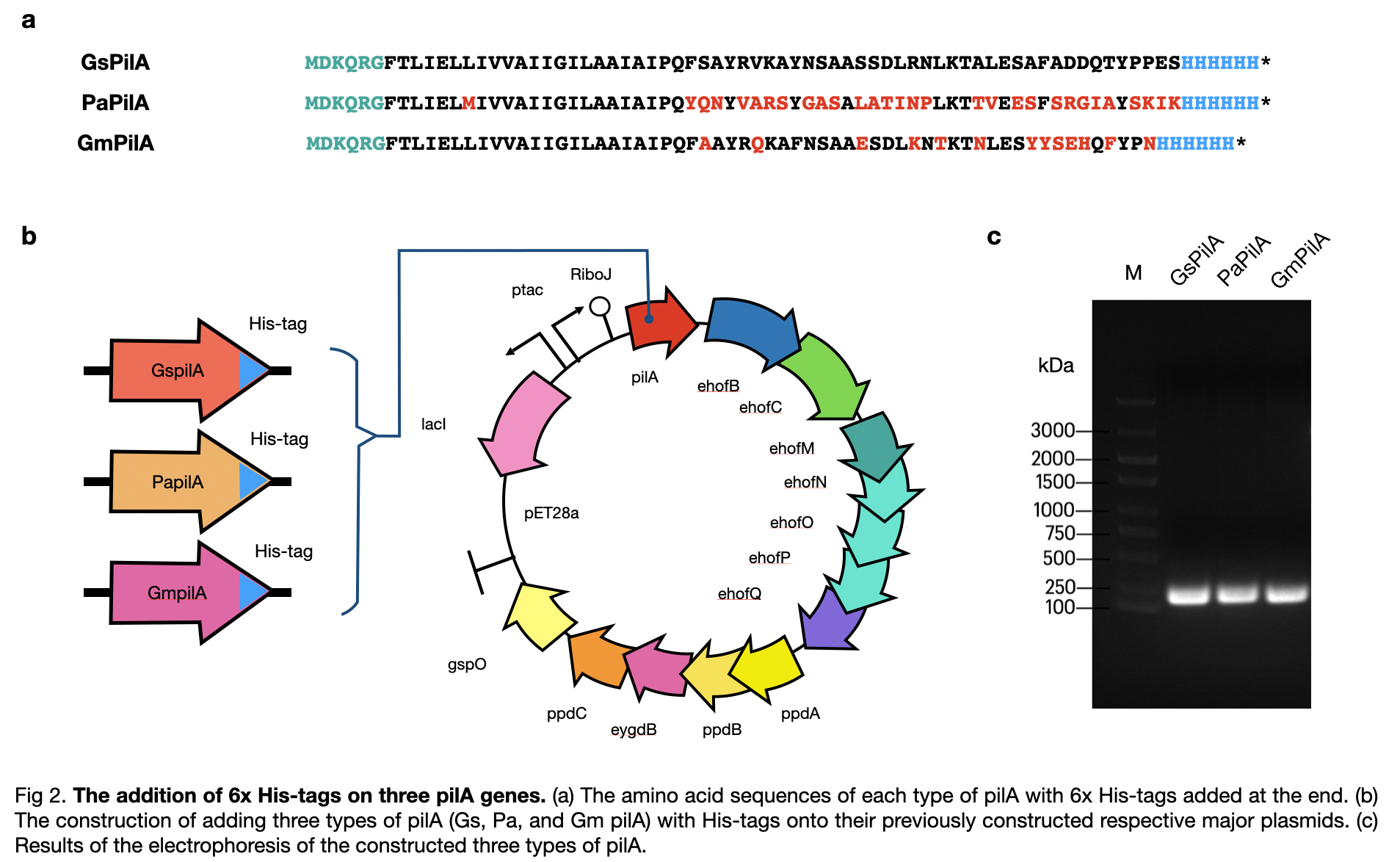
This year, LINKS_China constructed a conductive pilus, PaPilA, through slicing by overlap extension by PCR using 8 different primers to obtain a similar DNA sequence as in the Geobacter sulfurreducens, which produce the same amino acid sequence. To get a complete pili production system, we constructed the generator of the pili and obtained a complete circuit of BBa_K3552011 .
For pili expression, we chose E.coli BL21 because it is the best fit for our project as it has the highest pili yield and is easy to obtain. On the cultivation part, we cultured the bacteria in solid M9 mediums. Additionally, we provided the bacteria with glycerol as the carbon source because it was confirmed to help to improve the conductivity of the pili produced.
Protein production
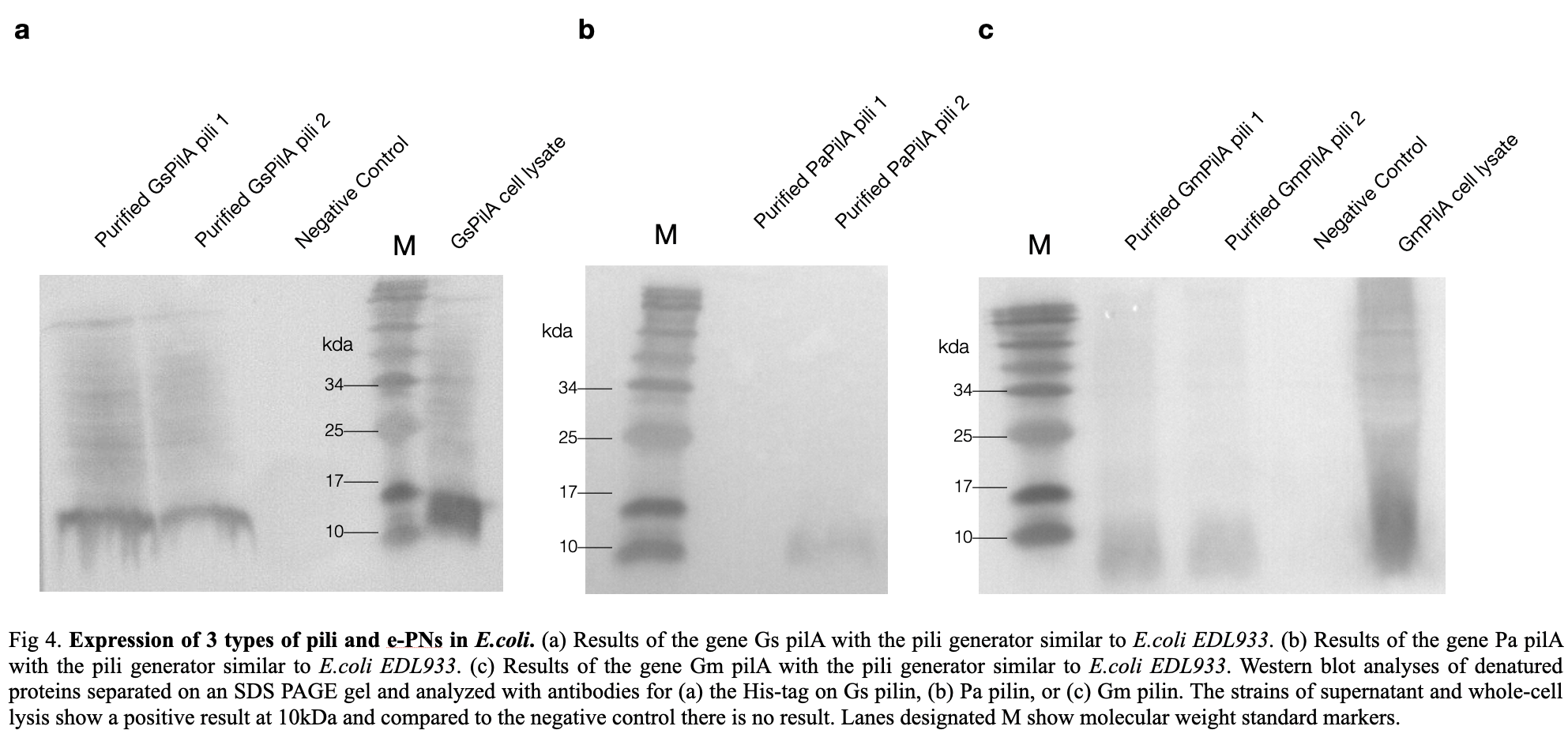
To confirm the E.coli produced the correct e-pili, we conducted a Western Blot experiment to convince the production. According to the positive strands on the membrane, we confirmed pili, with an expected molecular mass of 10kDa, was produced and purified successfully.
Conductiviity measure
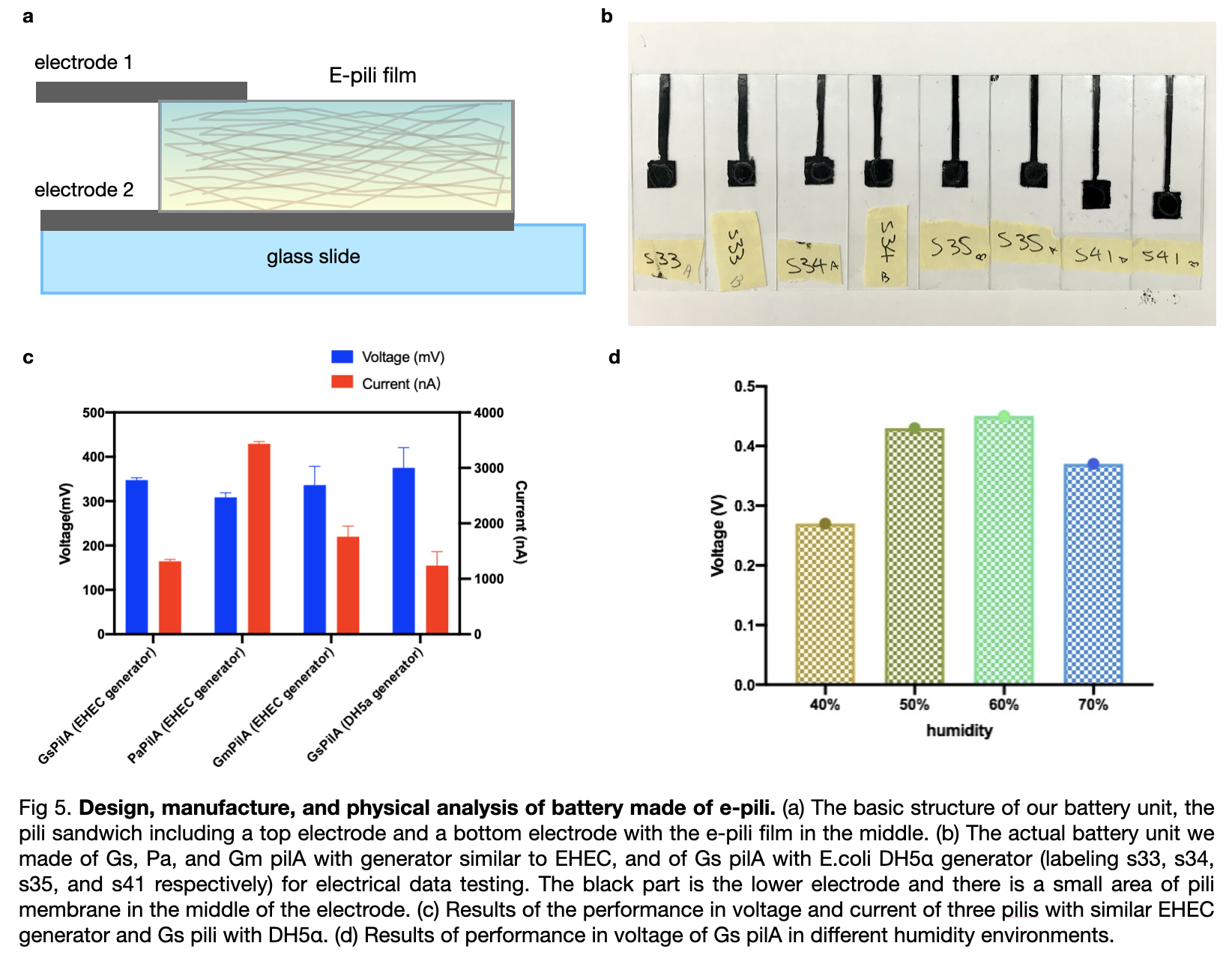
We manufactured the extracted pili into biological batteries and we also conducted experiments to measure the voltage of each battery. First, we manufactured six standard electrodes by using three pili, two pieces each, all with a triple layer of pili covered. Then we measured the voltage and compared the result which showed that Gm pili have the highest value of measured voltage while Gs pili have the least.
New measurement
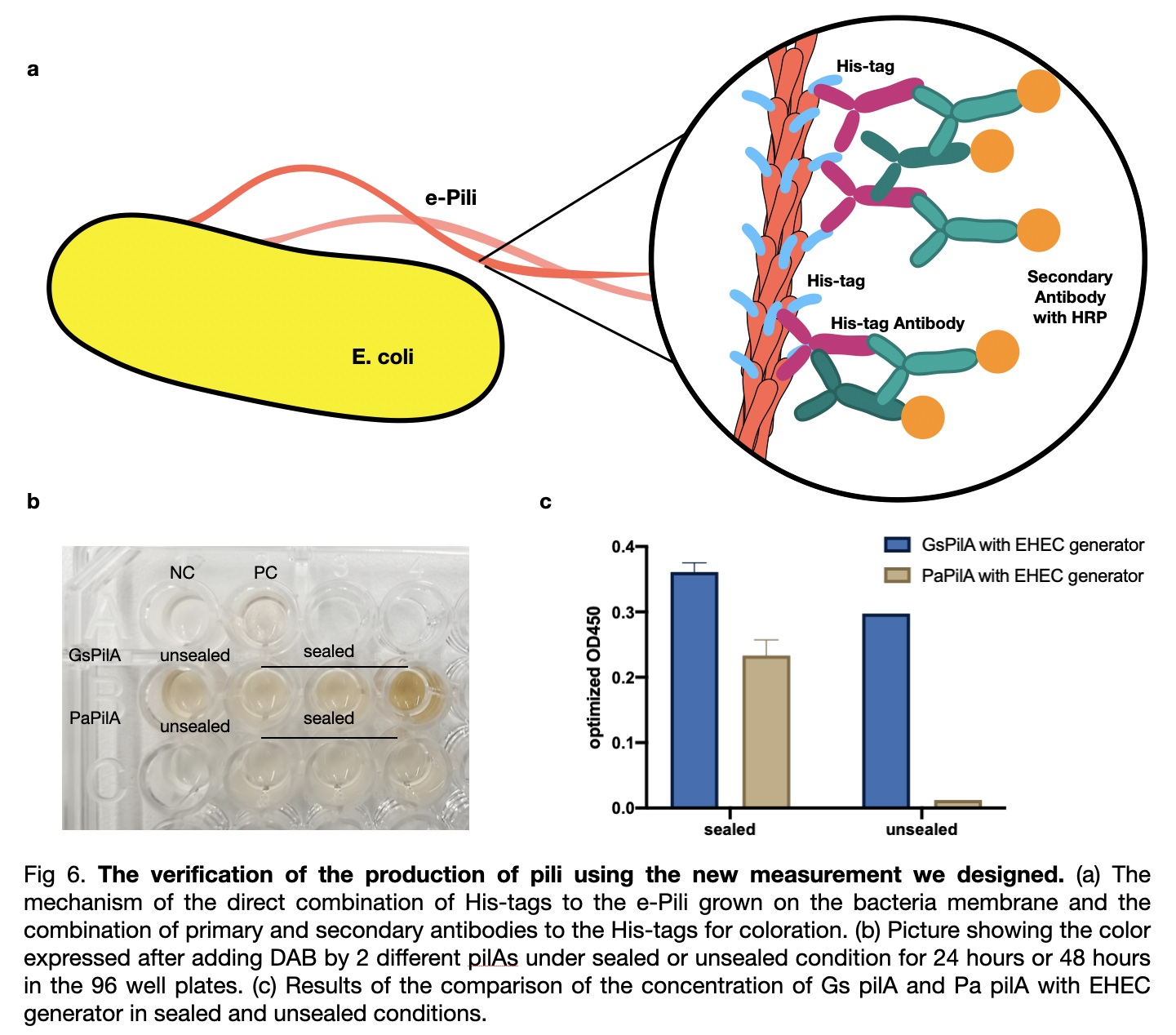
To compare the pili yield, we established a new measurement for a quicker, clearer, and more accurate determination of pili production. We used his-tags antibodies to attach to the his-tags on the pili, and then the secondary antibodies will be attached to the his-tags antibodies for coloration directly on the outer membrane of the bacteria. We analyzed the data and included the effects of two variables on pili production: cultivation time and mobile oxygen presence.
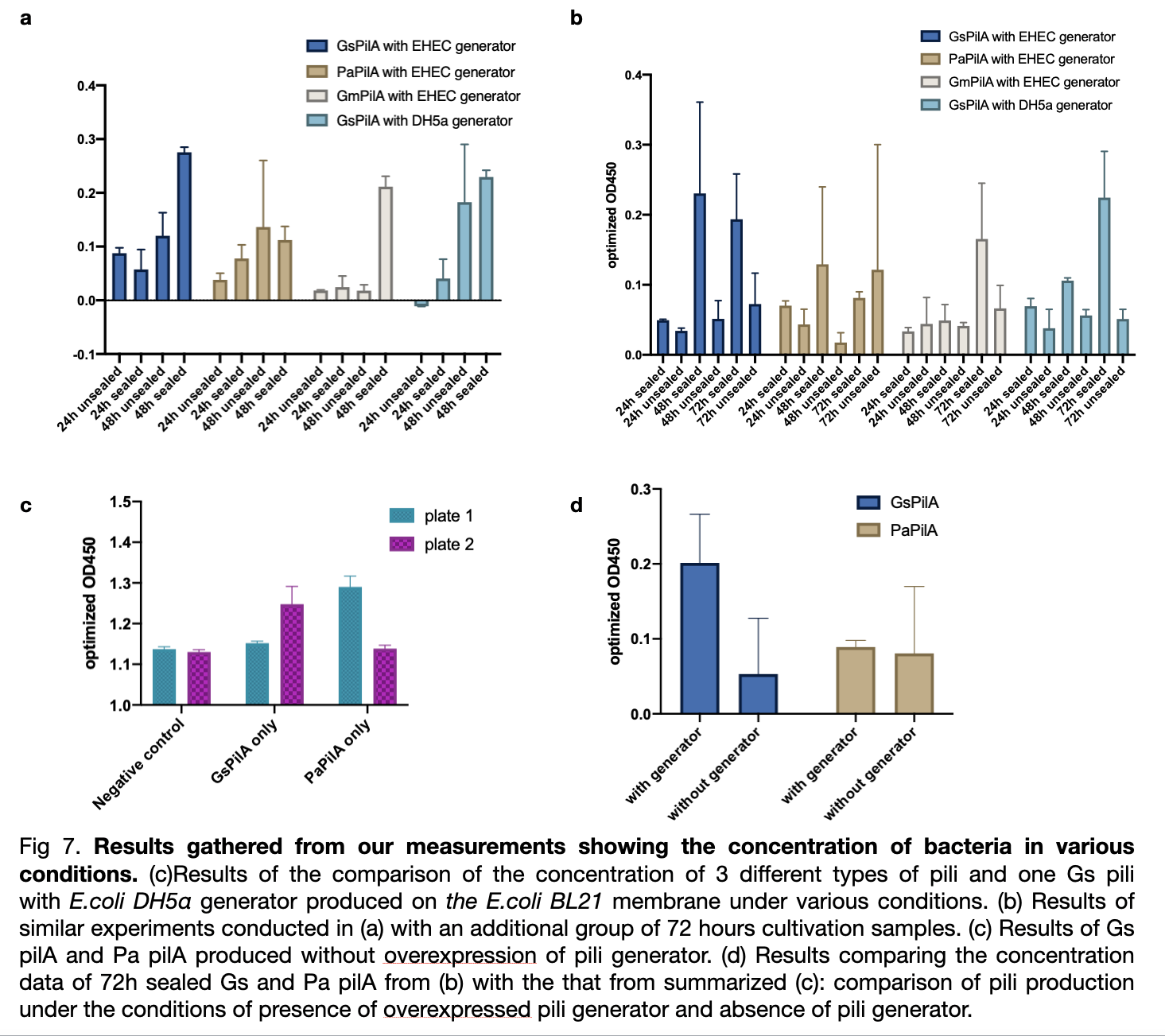
We measured the absorbance of all samples at od 450 divided by od 600 and subtract the value of negative control to obtain optimized od 450. The result shows a ranking of yield from the highest Gs pili to the lowest Gm pili. The trend for three experiments shows a smaller error bar for expression with EHEC similar generator. The sealed plates also received better results of higher production than the unsealed ones. The optimum cultivation time for GsPilA is 48 hours as it has an optimized od 450 value of 0.4 higher than 72 hours cultivation, whereas at 24 hours cultivation all kinds of bacteria expressed pili poorly.
| None |