Part:BBa_I712004
CMV promoter
a constitutive expression promoter for use in mammalian cells. Ribosome binding site is included.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Functional Parameters
| negative_regulators | -NA- |
| positive_regulators | -NA- |
[http://2009.igem.org/Team:Heidelberg Heidelberg 2009 iGEM team] characterized this promoter in Part:BBa_K203100 as a first application for its newly developed units of promoter strength in mammalian cells, [http://2009.igem.org/Team:Heidelberg/Project_Measurement Relative Expression Units (REU)] and [http://2009.igem.org/Team:Heidelberg/Project_Measurement Relative Mammalian Promoter Units (RMPU)], where RMPU is directly proportional to PoPs and measured on a RNA level, whereas REU is measured on the protein level. We found CMV to have a strength of 5,52 REU (Standard Error of the Mean = 0,60) in [http://2009.igem.org/Team:Heidelberg/Eucaryopedia#HeLa HeLa cells], 6,57 (SEM = 0,91) in [http://2009.igem.org/Team:Heidelberg/Eucaryopedia#MCF-7 MCF-7 cells] and 9,96 (SEM = 1,52) in [http://2009.igem.org/Team:Heidelberg/Eucaryopedia#U2-OS U2-OS cells] cells (Fig 1). We furthermore found that CMV is not a truly constitutive promoter (but we [http://2009.igem.org/Team:Heidelberg/Project_Measurement#There_are_no_truly_constitutive_promoters_in_mammalian_cells argue] that no such promoter exists). In [http://2009.igem.org/Team:Heidelberg/Project_Measurement RMPU] (a mRNA-based unit directly proportional to PoPs), CMV has an activity of approx. 2.89 RPMU in HeLa cells,as measured by qRT-PCR (Fig. 3)
 Fig. 1: Strength of the CMV promoter in different cell lines in REU. [http://2009.igem.org/Team:Heidelberg/Project_Measurement Relative Expression Units (REU)] were determined by three independent flow cytometry measurements on three different days. This measurement was confirmed by an independent technique - image analysis. |
 Figure 2: CMV strength changes depending on conditions As [http://2009.igem.org/Team:Heidelberg/Project_Measurement#Identification_of_challenges_unique_to_higher_eukaryotes there are no truly constitutive promoters in mammalian cells], both the reference promoter (Part:BBa_K203112) and CMV fluctuate dependent on condition (Erolimus induces extreme starvation). Measured by Flow cytometry 20 hours after transfection (unless specified otherwise). | |
 Fig. 3: Real-time RT-PCR data of CMV and JeT promoters.One group of HeLa cells were transfected with plasmid containing CMV promoter coupled to GFP. Another group with JeT promoter coupled to GFP was used as reference. RNA was extracted after 20 h and 50 h, followed by real-time RT-PCR. The Ct values were collected with a threshold of 0.05. The CMV activity compared to JeT at the same time point was calculated in MatLab as "arbitrary units" which correspond to amount of mRNA. |
The [http://2016.igem.org/Team:BostonU/Description 2016 BostonU iGEM team] further characterized this CMV promoter part by cloning it upstream of a GFP, transiently transfecting in HEK293FT cells, and assaying expression through flow cytometry. The part was cloned upstream of a GFP gene in a pSB1C3 backbone and transiently transfected in HEK293FT cells using PEI-mediated transfection.

As part of the characterization, this part was also directly compared to parts BBa_K1875016, and BBa_K1875018, created by the BostonU team as part of their project, Gemini. Parts BBa_K1875016 and BBa_K1875018 contain minimal CMV promoters and “guide operators” homologous to a 20 base pair guide RNA on a guide RNA expression vector. These new parts were co-transfected into HEK293FT cells with a dCas9-VPR and the complementary guide RNA expressing vector and then assayed using flow cytometry. Fluorescence of the CMV promoter device was measured relative to these devices.
The CMV promoter device successfully expressed GFP in HEK293FT cells. Part BBa_K1875016, the operator containing only one binding site for the dCas9-VPR, expressed GFP at a level lower than the CMV promoter while part BBa_K1875018 , the operator containing three binding sites, had higher GFP expression.
The experimental procedures used in this assay involved measuring fluorescence using Mean Fluorescence Intensity (M.F.I). Thus, the absolute values are arbitrary units, and cannot be directly compared to other systems. Our experiment, however, does reveal the relative strength of the CMV promoter device as compared to both of our well-characterized parts.
Improvement from iGEM 2018 Team Nanjing_NFLS
This year, we Nanjing_NFLS have improved the previous part BBa_I712004 by changing CMV’s second natural NF-kB binding site into high-affinity SELEX-selected artificial sequence GGGGATTCCC. We evaluated the optimized promoter activity with EGFP, and evaluated the promoter in various cells with the Dual-Luciferase reporter assay and Gluc reporter assay. The results revealed mut CMV BBa_K2597003 we created showed higher transcriptional activity compared to wt CMV. See more details here https://parts.igem.org/Part:BBa_K2597003
MIT-2019 Characterization
While the majority of our project was focused on engineering leader cells, we were also interested in manipulating the follower cells by genetically engineering HL-60 cells. We noticed that while there were several methods of transfection described for HL-60 cells ( including a project by the <a href=”http://2009.igem.org/Team:UCSF”>2009 UCSF iGEM team</a>, a paper by <a href=”http://limlab.ucsf.edu/papers/pdfs/park_2014.pdf”>Park et. al.</a>), we did not find any systematic data on the function of commonly used promoters in this cell type. Considering that HL-60 cells are relatively difficult to transfect and require harsh transfection conditions (electroporation) that can result in cell death and low transfection efficiency, we wanted to find a promoter that would lead to reliable and strong expression of transfected genes in order to facilitate our future experiments with the SynNotch system and engineering of leader cells to become followers.
In particular, we characterized the expression of the fluorescent proteins EYFP and TagBFP encoded on plasmids under the CMV and hEF1a promoters and transfected by electroporation into undifferentiated HL-60 cells.
Figure 1:

Figure 2a:

Figure 2b:

We observed a lot of cell death due to electroporation. Events from the flow cytometry analysis were first plotted on an FSC/SSC dot-plot graph to set an analysis gate, as shown in Figure 1. For the cells within the analyzed gate we looked at fluorescence in the FITC channel (excitation 488 nm, detection window 530/30 nm) for detection of EYFP and Pacific Blue channel (excitation 405 nm, detection window 450/50 nm) for detection of TagBFP. We found that 52% of cells transfected with CMV-EYFP were fluorescent in the yellow channel, and 35% of cells transfected with CMV-TagBFP were fluorescent in the blue channel. On the other hand, only 1.5% of cells transfected with hEF1a-EYFP and 8% of cells transfected with hEF1A-TagBFP were weakly fluorescent.
Figure 2 shows the overlay of histograms for untransfected cells (control, shown in green) and cells transfected with the fluorescent protein encoded under a CMV promoter (shown in blue) or hEF1a promoter (shown in red) for a) TagBFP and b) EYFP.
Improvement from the iGEM-team Hannover 2020
Overview
As an improvement of the natural CMV-promoter that shows no activation following LPS-stimulation, we designed a promoter based on the CMV-promoter that displays high activation after LPS-stimulation. Shortly, we replaced the CMV-enhancer and large parts of the promoter by NF-κB and AP1 binding sites just leaving the TATA-box and the Initiator-Sequence of the original CMV-promoter. The improved part and a full description of the improvement can also be found here (BBa_K3338002). Using the same approach, we have developed a promoter that shows 25-fold basal expression of the CMV enhancer/promoter named Synthetic promoter 1 (BBa_K3338007).
Motivation
One of the central aspects of our inflammatory toxin sensor is the LPS-sensitivity of its promoter. Because of that, one of our goals was to design a LPS-sensitive promoter showing high induction following LPS-treatment. In a previous study it was shown that the human CMV immediate‐early (CMV-IE) gene enhancer/promoter shows NF-κB and c-Jun dependent regulation in response to LPS and bacterial CpG‐oligodeoxynucleotides (Lee et al. 2004). The experiments were performed in the macrophage cell line RAW 264.7 and showed that a maximum of CMV induction was reached 6 h after LPS-supplementation followed by a decrease in promoter activity (Lee et al. 2004). This observation was not reproducible in HeLa-cells using a CMV-hGLuc construct (BBa_K3338017) in the mammalian expression vector pEGFP-C2 (see figure 1).
 Figure 1: Relative activity of CMV promoter in HeLa cells 24 hours (A) and 48 hours (B) after treatment with different concentrations of LPS for 3 hours, normalized to untreated control (0 µg/mL LPS). Data shown represents mean ± SEM of n=4 biological replicates. Statistical analysis was performed by unpaired t-test in comparison to untreated control, significance level: 10 %, significance is indicated by asterisk.
Figure 1: Relative activity of CMV promoter in HeLa cells 24 hours (A) and 48 hours (B) after treatment with different concentrations of LPS for 3 hours, normalized to untreated control (0 µg/mL LPS). Data shown represents mean ± SEM of n=4 biological replicates. Statistical analysis was performed by unpaired t-test in comparison to untreated control, significance level: 10 %, significance is indicated by asterisk.
To achieve high induction of the CMV-promoter following LPS-treatment of the cells, we had to modify the promoter/enhancer-regions of the CMV-promoter.
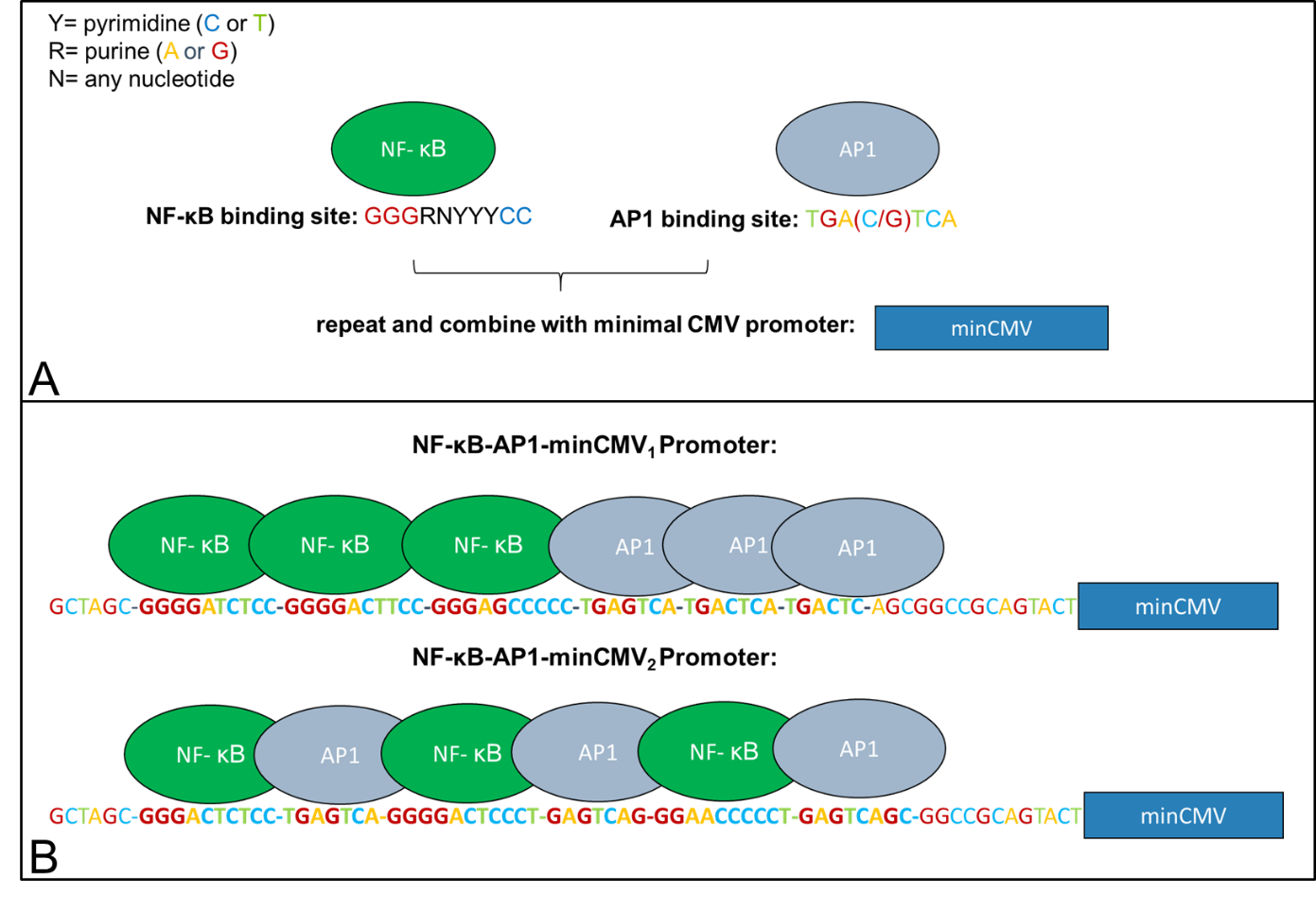
Part Design
LPS-sensitivity of the CMV-promoter is mediated through NF-κB and AP1 transcription factor binding sites (Lee et al. 2004). In order to abolish effects of other transcription factors on promoter activity, the CMV-enhancer and large parts of the promoter were removed just leaving a minimal CMV promoter with the TATA-box and the initiator-sequence which are essential for promoter activity. To make the promoter sensing, we added AP1 and NF-κB transcription factor binding sites right in front of the CMVmin promoter. These sequences were generated using a MATLAB tool which creates random AP1 and NF-κB sites based on the consensus sequence “GGGRNYYYCC” for NF-κB and “TGAXTCA” for AP1, where Y is C or T, R is A or G, X is C or G and N is any base (Chen et al. 1998, Alam and Den 1992). Two variants were built (see figure 2). One exhibiting three NF-κB sites followed by three AP-1 sites (Synthetic promoter_1, BBa_K3338007) and another one consisting of three NF-κB/AP-1-sites (Synthetic promoter_2, BBa_K3338002).
Results
In order to test the synthetic promoters described above, their activity following LPS-stimulation was ascertained using hGLuc as a reporter gene. Therefore SynthP_1-hGLuc (BBa_K3338021) and SynthP_2-hGLuc (BBa_K3338022) were cloned into pEGFP-C2 using AseI/HindIII to linearize the vector (CMV-enhancer/promoter and EGFP were removed). The constructs were transfected in HeLa cells. The cells were treated with LPS for 3 h and hGLuc expression was assessed 24 h and 48 h after LPS-treatment. Figure 3 and figure 4 show quantitative data of the experiments.
 Figure 3: Relative activity of NF-κB-AP1-minCMV1 promoter in HeLa cells 24 hours (A) and 48 hours (B) after treatment with different concentrations of LPS for 3 hours, normalized to untreated control (0 µg/mL LPS). Data shown represents mean ± SEM of n=4 biological replicates. Statistical analysis was performed by unpaired t-test in comparison to untreated control, significance level: 10 %, significance is indicated by asterisk.
Figure 3: Relative activity of NF-κB-AP1-minCMV1 promoter in HeLa cells 24 hours (A) and 48 hours (B) after treatment with different concentrations of LPS for 3 hours, normalized to untreated control (0 µg/mL LPS). Data shown represents mean ± SEM of n=4 biological replicates. Statistical analysis was performed by unpaired t-test in comparison to untreated control, significance level: 10 %, significance is indicated by asterisk.
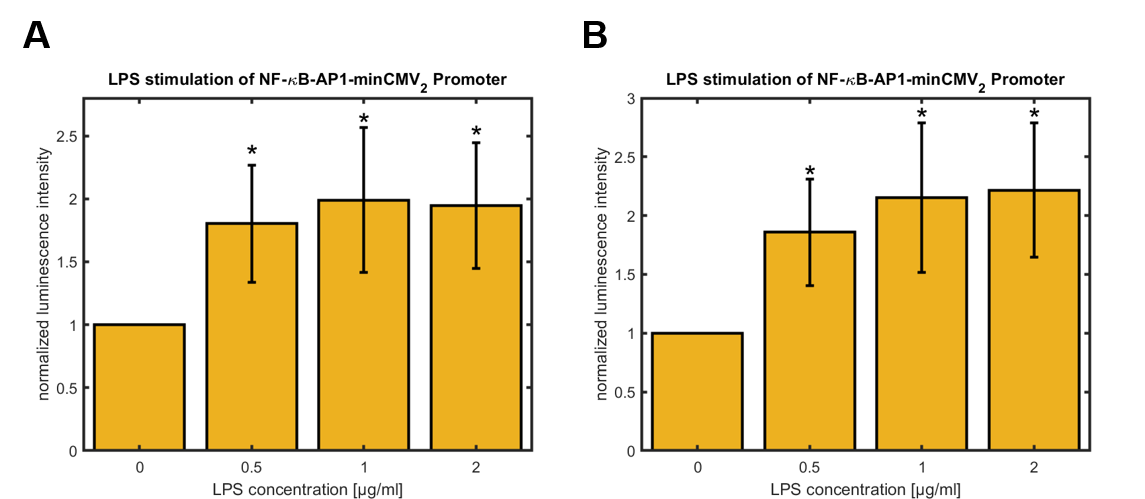 Figure 4: Relative activity of NF-κB-AP1-minCMV2 promoter in HeLa cells 24 hours (A) and 48 hours (B) after treatment with different concentrations of LPS for 3 hours, normalized to untreated control (0 µg/mL LPS). Data shown represents mean ± SEM of n=4 biological replicates. Statistical analysis was performed by unpaired t-test in comparison to untreated control, significance level: 10 %, significance is indicated by asterisk.
Figure 4: Relative activity of NF-κB-AP1-minCMV2 promoter in HeLa cells 24 hours (A) and 48 hours (B) after treatment with different concentrations of LPS for 3 hours, normalized to untreated control (0 µg/mL LPS). Data shown represents mean ± SEM of n=4 biological replicates. Statistical analysis was performed by unpaired t-test in comparison to untreated control, significance level: 10 %, significance is indicated by asterisk.
For both synthetic promoters a significant upregulation of the promoter activity was observed 24 h and 48 h after LPS-treatment of the cells for 3 h. Whereas the activity of the synthetic promoter_1 maximally increases about 40 % over the activity of the untreated cells, the activity of the synthetic promoter_2 rises up to approximately 100 % over the basal activity. For both promoters, the highest values were observed for cells treated with 2 μg/mL LPS. Furthermore, the basal activities of both synthetic promoters were compared (see figure 5) using the basal activity of the CMV promoter as a standard (see figure 5).
 Figure 5: Relative basal activity of all tested promoters in HeLa cells 48 hours (A) and 72 hours (B) after transfection. Data was normalized to CMV promoter which served as reference. Data shown represents mean ± SEM of n=4 biological replicates.
Figure 5: Relative basal activity of all tested promoters in HeLa cells 48 hours (A) and 72 hours (B) after transfection. Data was normalized to CMV promoter which served as reference. Data shown represents mean ± SEM of n=4 biological replicates.
The results indicate that both synthetic promoters exhibit a higher basal activity than the original CMV-promoter. This could be due to higher transfection efficiencies as compared to the original CMV-hGLuc construct because of the lesser plasmid size. However, further experiments are needed to clarify. The naturally occurring LPS-sensitive human IL-6 promoter shows a basal activity comparable with the CMV-promoter. For the usage as a LPS-sensor the promoter ideally exhibits a low basal activity like the IL-6 promoter. But even more important is its LPS sensitivity. Figure 6 indicates that the gain of IL-6 activity is significant but much smaller compared to the synthetic promoters.
 Figure 6: Relative activity of IL6 promoter in HeLa cells 24 hours (A) and 48 hours (B) after treatment with different concentrations of LPS for 3 hours, normalized to untreated control (0 µg/mL LPS). Data shown represents mean ± SEM of n=4 biological replicates. Statistical analysis was performed by unpaired t-test in comparison to untreated control, significance level: 10 %, significance is indicated by asterisk.
Figure 6: Relative activity of IL6 promoter in HeLa cells 24 hours (A) and 48 hours (B) after treatment with different concentrations of LPS for 3 hours, normalized to untreated control (0 µg/mL LPS). Data shown represents mean ± SEM of n=4 biological replicates. Statistical analysis was performed by unpaired t-test in comparison to untreated control, significance level: 10 %, significance is indicated by asterisk.
Conclusions
Here we describe BBa_K3338002 as an improved CMV-promoter that is capable of detecting LPS in the surrounding of the cell via the TLR4-NF-κB/c-Jun pathway leading to enhanced gene expression. It is much more sensitive than the naturally occurring LPS-sensitive interleukin-6 promoter but shows a higher basal activity than the IL-6 and the CMV-promoter. Regarding the basal activity and LPS-sensitivity the Synthetic promoter_2 has tremendous advantages over Synthetic promoter_1 making it the better LPS-sensor. But it is of note that the basal activity of the Synthetic promoter_1 is up to approximately 25-times higher than of the natural CMV-promoter. This tremendous enhancement of promoter activity makes it applicable for protein expression in mammalian expression systems when maximum protein quantities are required.
References
Alam, J., & Den, Z. (1992). Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. The Journal of biological chemistry, 267(30), 21894–21900.
Chen, F. E., Huang, D. B., Chen, Y. Q., & Ghosh, G. (1998). Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature, 391(6665), 410–413.
Lee, Y., Sohn, W. J., Kim, D. S., & Kwon, H. J. (2004). NF-kappaB- and c-Jun-dependent regulation of human cytomegalovirus immediate-early gene enhancer/promoter in response to lipopolysaccharide and bacterial CpG-oligodeoxynucleotides in macrophage cell line RAW 264.7. European journal of biochemistry, 271(6), 1094–1105.
//direction/forward
//chassis/eukaryote
//promoter
//regulation/constitutive
| negative_regulators | |
| positive_regulators |



