Part:BBa_K3147010
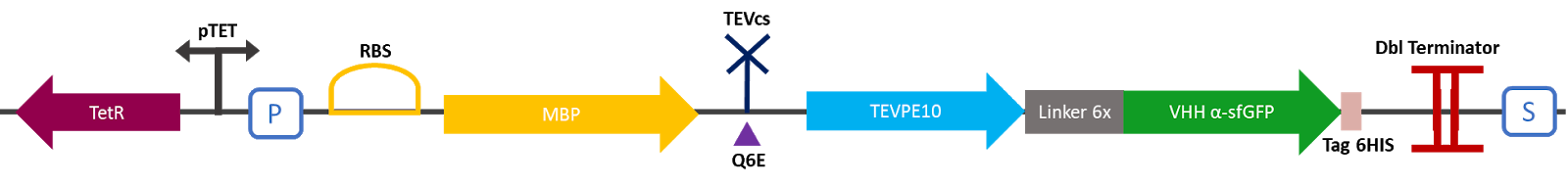
I : parts BBa_K3147010 (MBP-TEVcs-TEV) function :
The Montpellier 2019 team made this construction in order to be able to compare the basal activity of the TEVPE10 protease with the same fused protease to a nanobody type VHH. This construction produces an MBP fused to a TEV protease attached to a specific VHH of the sfGFP. Between the MBP and the protease a modified TEV cutting site is added. MBP increases the solubility of the fusion protein [1] preventing the aggregation of the protein of interest, this stabilizes the expression, the sequence of the produced MBP does not have a signal peptide which enables to keep the protein in the cytosol. The TEV cutting site allows to separate MBP from TEV once the protein is produced, indeed the TEV protease is capable of self-cleavage to remove MBP [2]. An affinity tag 6 histidine is added in C-ter of the VHH.
Figure 1 : Construct Design: MBP-TEVcs-TEV-VHH with modified TEV cutting site. The objective of the construction is to be able to compare the activity of a TEV protease alone against a TEV protease fused to a VHH.
II. Proof of function
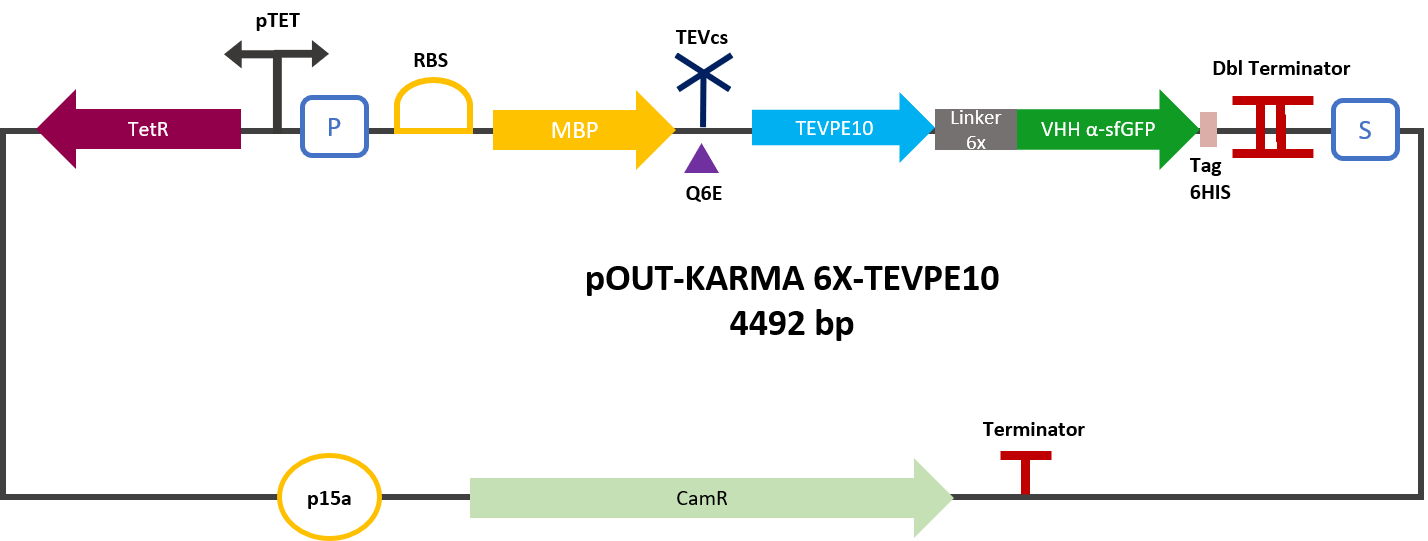
Figure 2: MBP-TEVPE10-VHH reporter gene in its backbone pOUT18.
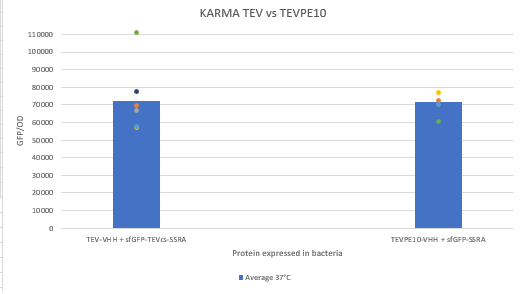
The experimental approach to test the activity of the protease is to compare the fluorescence restoration rate of sfGFP against MBP-TEV-VHH and MBP-TEVPE10-VHH. In this experiment, basal controls of maximum and minimum fluorescence of reporter genes were used. Fluorescence data are obtained by Plate reader. The construction was cloned by Gibson Assembly in a backbone pOUT18 under the control of a TET ON promoter, in order to control its expression. The protease is expressed by inducing the TET promoter with 50ng/mL of aTc (anhydrotetracycline).
Figure 3 : Comparison of the activity of VHH-TEVPE10 to the VHH-TEV WT by measuring the fluorescence of a reporter that needs to be cutted from a proteolysis tag
Reference
[1] Raran-Kurussi, Sreejith, et David S. Waugh. 2012. « The Ability to Enhance the Solubility of Its Fusion Partners Is an Intrinsic Property of Maltose-Binding Protein but Their Folding Is Either Spontaneous or Chaperone-Mediated » éd. Bostjan Kobe. PLoS ONE 7(11): e49589.
[2] Shih, Y.-P. 2005. « Self-Cleavage of Fusion Protein in Vivo Using TEV Protease to Yield Native Protein ». Protein Science 14(4): 936‑41.
[3] Yi, L. et al. 2013. « Engineering of TEV Protease Variants by Yeast ER Sequestration Screening (YESS) of Combinatorial Libraries ». Proceedings of the National Academy of Sciences 110(18): 7229‑34
[4] Kubala, M. H., Kovtun, O., Alexandrov, K., & Collins, B. M. (2010). Structural and thermodynamic analysis of the GFP:GFP-nanobody complex. Protein science : a publication of the Protein Society, 19(12), 2389–2401. doi:10.1002/pro.519
| None |