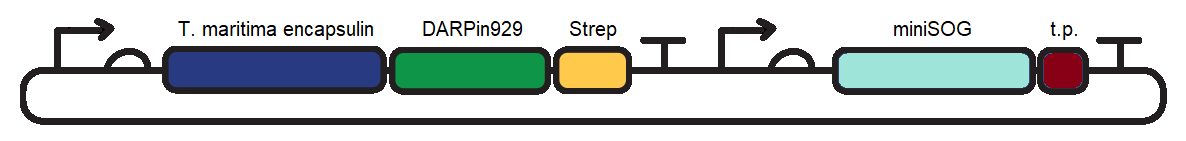
Part:BBa_K3111502
TmEncH_DARPin929_StrepII + miniSOG_TP
T. maritima encapsulin (6-His) and DARPin929 fusion protein co-expressed with miniSOG in order to report targeting of HER2+ cells and cause a cytotoxic effect in them upon illumination.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 171
Illegal BglII site found at 586
Illegal BamHI site found at 950
Illegal BamHI site found at 2101 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1677
Illegal BsaI.rc site found at 2321
Illegal SapI.rc site found at 520
Illegal SapI.rc site found at 551
Composite Part Design
T. maritima encapsulin surface display and cargo loading were previously proven in BioBricks BBa_K3111501 and BBa_K3111402. In these experiments we looked at how we can use these properties for the construction of a drug delivery platform.
To induce cytotoxic action, we proceeded with loading of the encapsulin system with a fluorescent photosensitiser protein, miniSOG (BBa_K3111032). Under blue-light illumination, miniSOG is activated and generates reactive oxygen species which are damaging to cancer cells. To achieve this we cloned BBa_K3111501 and BBa_K3111302 together to construct the plasmid observed in Figure 1.
Our ultimate aim was to test whether this device can illicit cytotoxic effect in SK-BR-3 cells with two layers of selectivity: DARPin binding and light induction.
Experimental Results
Loading cytotoxic cargo
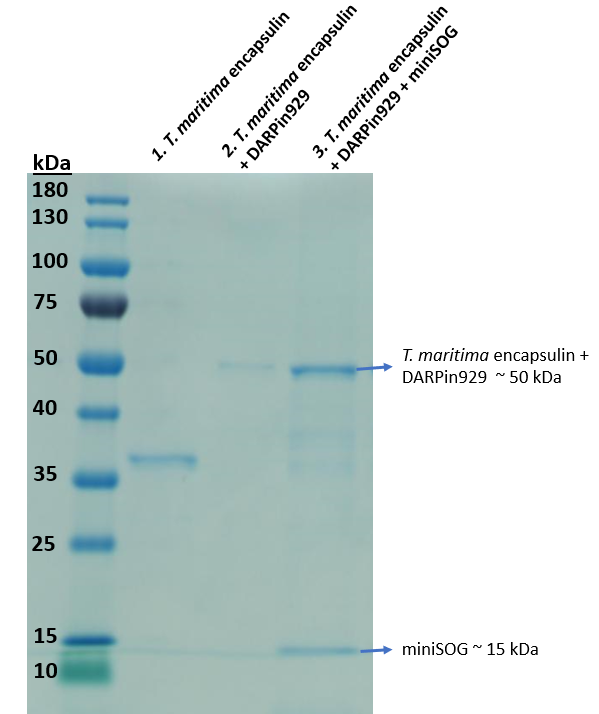
To enable cargo loading we utilised a short native C-terminal targeting peptide (1) that was previously described to allow foreign cargo loading. Thus, miniSOG was fused to the C-terminal targeting peptide downstream via a flexible linker. Once cloning was proven successful, we used BL21 (DE3) cells for protein expression in a 50mL shake flask culture. Then we purified the soluble cell lysate and obtained a sample to run on an SDS-PAGE to investigate cargo loading.
In Figure 2, we confirmed the assembly of the multicomponent drug delivery platform. Comparing the 2nd and 3rd lane, an additional band of 15 kDa was observed. MiniSOG does not possess a StrepII-tag as observed in Figure 1, thus it would not elute unless it was bound to the inside of an assembled T. maritima encapsulin, confirming loading. Further fluorescence spectrophotometric analysis also indicated fluorescence compared to non-loaded encapsulins.
Binding and cytotoxicity assay
Once the construction of the multicomponent drug delivery vehicle was proven successful through SDS-PAGE and fluorescent spectrophotometric analysis we proceeded with mammalian cell culture studies. We used SK-BR-3 breast adenocarcinoma cells that are overexpressing HER2 receptor, which we had prieviously shown to be targeted by DARPin929 (BBa_K3111011).
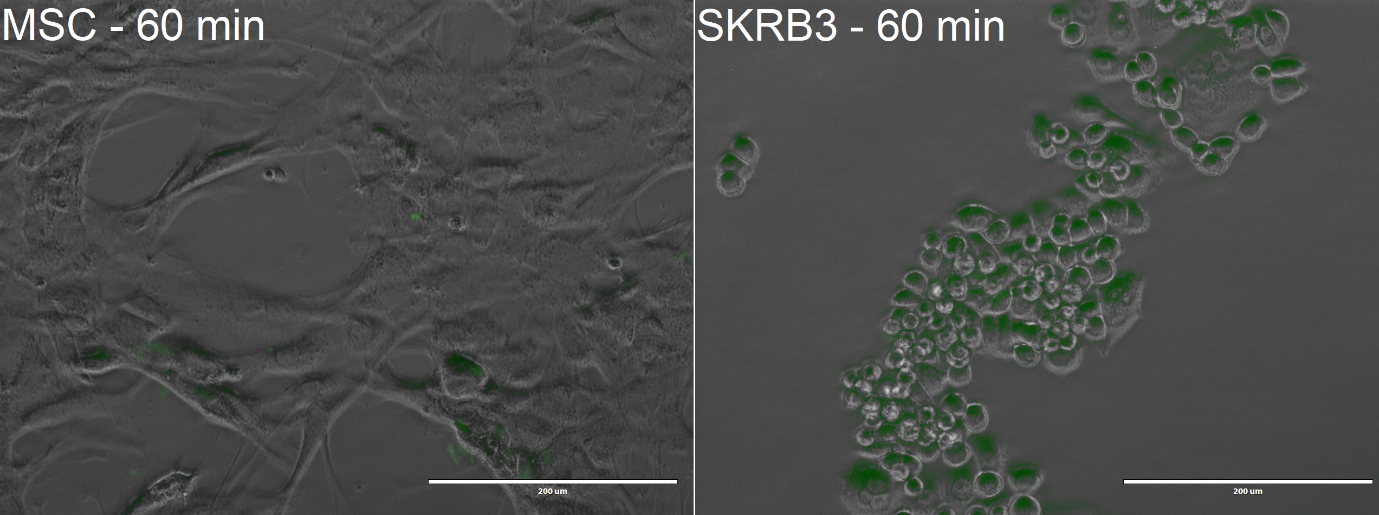
The cells were first incubated with the Encapsulin-DARPin929-miniSOG drug delivery construct for 30 min, then they were illuminated with white light at 40 lumens/cm2 for 30 mins in order to activate the cytotoxic effect of the encapsulated miniSOG at 37 °C and 20% oxygen. At the end of the experiment, the cells were visualised with EVOS FL microscope to observe uptake of the encapsulins. Following that, all the cell samples were stained using the Annexin V – Propidium Iodide staining kit for determination of potential cell death and percentage loss in viability using flow cytometry. Moreover, we wanted to examine the specificity of the cytotoxic effect thus we replicated the experimental set-up and conditions with Mesenchymal Stem Cells (MSCs), which do not possess the HER2 receptor. We also included two controls which comprised of the SK-BR-3 and MSCs alone.
From the confocal microscopy imaging in Figure 3, we could see that after 1-hour incubation green fluorescence from miniSOG was detected in both samples. Fluorescence was not expected for the MSCs, since from our previous experiments with BBa_K3111201 we have not observed any unspecific binding. We hypothesise that the concentration of construct that we loaded was too high, thus non-specific passive uptake was initiated. However, since significantly more fluorescence was observed in SK-BR-3 cells, we can deem that the system is indeed selective.
The plots in Figure 4 are separated in 4 quadrants over which the cell population is distributed. The upper left quadrant indicated the percentage of necrotic cells, the upper right the percentage of late apoptotic cells, the lower left shows the percentage of viable cells and the lower right the percentage of early apoptotic cells. Cell controls in Figure 4 (a) and (d) helped to define the normal boundaries for their respective cell type
From Figure 4 (e) we could observe a shift in cell population majorly towards the lower right quadrant indicating early apoptosis of the cell before illumination compared to our control cell population in 4 (d). While this was not expected, we speculate that light falling on the well plates during sample processing could have induced miniSOG, thus resulting in a loss of cell viability. MSCs however presented only a minimal percentage of early apoptotic cells. After illumination, we observed a considerable shift towards the upper and lower right quadrants in Figure 4 (f), indicating a high rate of apoptosis with a total of around 70% reduction in cell viability. The loss of viability in MSC through early apoptosis observed in Figure 4 (c) could be associated to the greater sensibility of stem cells to environmental condition fluctuation (in this instance, strong illumination) or the handling of the cells required for imaging and staining.
To conclude, these experiments show that our drug delivery platform successfully targets HER2 overexpressing SK-BR-3 cells and reduces their viability at a significantly higher level than MSCs once illuminated with light, thus demonstrating double selectivity in terms of cytotoxicity.
| None |