Part:BBa_K2812000
Carbohydrate-binding domain from MpIBP
Carbohydrate binding domain from the Marinomonas primoryensis ice-binding protein (MpIBP). It requires millimolar concentrations of calcium ions to properly fold into a globular β-fold. A coordinated calcium ion is used to bind sugar moieties, such as glucose. Unfolding of the domain can be induced by the addition of EDTA, which chelates the calcium ions and prevents binding of sugar moieties. It does not require the presence of other domains to fold and therefore it can be used as a modular protein domain to bind sugar moieties. TU Eindhoven 2018 used the carbohydrate binding domain from MpIBP to tether E. coli bacteria to dextran, a glucose-based polymer.
Usage and Biology
The Marinomonas primoryensis ice-binding protein (MpIBPis a 1.5-MDa adhesin used by the Antarctic bacterium M. primoryensis to bind to ice and diatoms to position itself on top of the water column to access nutrients and oxygen. The carbohydrate binding domain from this protein is used to bind extracellular polysaccharides to form microcolonies and to tether M. primoryensis to other photosynthetic microorganisms.
Characterisation by TU Eindhoven (2018)
Cloning
The biobrick has been characterized by the iGEM 2018 Eindhoven team. The carbohydrate binding domain was synthesized by IDT. Both the linearized plasmid backbone pSB1C3 and the carbohydrate binding domain were double digested by EcoRI and PstI and subsequently assembled via ligation after purification of the backbone. Succesful transformation of the ligated product into Novablue E. coli cells was followed by a colony PCR using the VF2 and VR primers, to confirm the that the desired insert has been inserted into pSB1C3. The results of the colony PCR can be seen on the right. Due to synthesis problems at IDT, the construct delivered contained multiple DNA fragments that could not be separated, explaining the large amount of colonies with a wrong insert. The insert in lane 10 (yellow box), has the correct length and was used for further sequencing. Sanger sequencing of this DNA confirmed the desired insert has been incorporated in pSB1C3.
Proof of Functionality
Experimental Setup
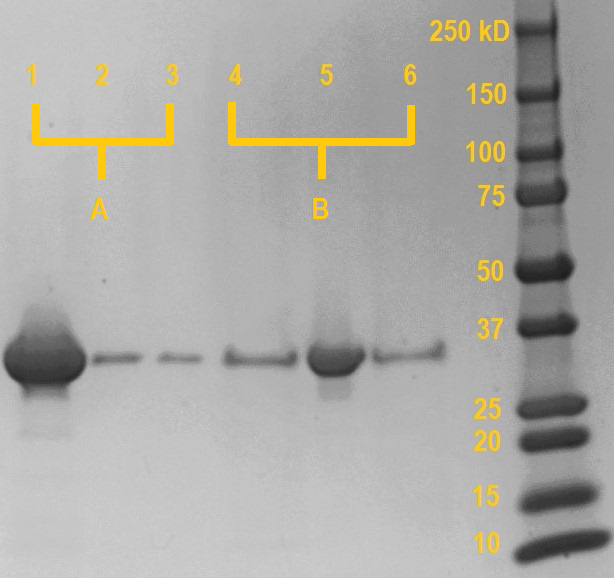
This experiment was performed to verify the calcium-dependent carbohydrate binding capabilities of the MpIBP carbohydrate binding domain. The MpIBP carbohydrate binding domain was expressed, isolated and purified from E. coli BL21 (DE3). Equimolar amounts purified MpIBP carbohydrate binding domain were incubated with Superdex dextran beads in the presence of 5mM CaCl2 (sample A) or 10mM EDTA (Sample B). Dextran is a glucose polymer, to which our MpIBP carbohydrate binding domain should bind in the presence of millimolar concentrations of calcium. After incubation, each sample was centrifuged shortly at 8,000 rpm to pull the dextran beads (and any dextran-bound proteins) down from the mixture and the supernatant was isolated. Next, the beads were resuspended in buffer without CaCl2 (sample A) or with EDTA (sample B), allowed to incubate. After incubation, the samples were centrifuged shortly at 8,000 rpm and the supernatant was isolated.
SDS samples were prepared from the dextran beads after the final centrifugation (lane 1 & 4), supernatant from the first incubation (lane 2 & 5) and supernatant from the resuspension (lane 3 & 6). The samples were boiled at 95 degrees Celcius for 15 minutes and the SDS gel was run at 120V for one hour. Anything bound to the dextran beads will be released into the solution during the boiling.
Results
As can be seen on the SDS gel on the right, if the MpIBP carbohydrate binding domain is incubated with dextran in the presence of millimolar concentrations of calcium, only a very small amount of protein remains unbound in the supernatant (lane 2; probably due to saturation of the dextran beads). However, if the protein domain is incubated in the presence of EDTA, which chelates the calcium, almost all protein remains in unbound in the supernatant (lane 5).
In the subsequent washing step (lane 3 & 6), aspecifically bound protein is washed away from the beads using buffer devoid of calcium. For both sample A and B, the amount of aspecifically bound protein released during the washing is very minor and comparable. This shows that most proteins stay bound in the case of sample A, while in sample B most protein has already been removed in the previous step.
Boiling the dextran beads releases any protein still bound after washing. In the case of sample A (initially incubated in the presence of calcium), a very large amount of protein has remained bound during the washing and is released from the beads (lane 1). Conversely, the beads incubated with the protein in the presence of EDTA release negligible amounts of protein compared to sample A, as almost all protein has already been washed away.
Conclusion
The observations above show that the binding of the MpIBP carbohydrate binding domain is calcium dependent and that negligible binding occurs in the absence of calcium. Once bound in the presence of calcium, there is no need for additional calcium to remain present in the solution as the protein-carbohydrate complex holds on to the calcium ion tightly. EDTA can be used to chelate the calcium, inducing unfolding and prevent binding of the protein.
The isolated carbohydrate binding domain of the 1.5 MDa MpIBP has been proven to retain its functionality in the absence of the rest of the adhesin.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
//collections/biofilm
//proteindomain
//proteindomain/affinity
//proteindomain/binding
| None |