Part:BBa_K1926031
The SNAP-mCHERRY- CYAN UNIT
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 419
- 1000COMPATIBLE WITH RFC[1000]
This part is the SNAP-mCHERRY-CYAN UNIT of cyclebow including a SNAP UNIT, a mCHERRY UNIT and a plain CYAN. The SNAP UNIT, which can be cut off by CRE, includes an sv40 nuclear location sequence (NLS), a covalent labeling of fusion proteins named SNAP-tag® and an sv40 terminator flanked by homodromous recognition site of recombinase CRE named loxP. The mCHERRY UNIT, which can be cut off by VIKA, includes a degradation tag named DBOX, a fluorescent gene mCherry and an sv40 terminator flanked by homodromous recognition site of recombinase VIKA named Vox.
Usage
You may use this part to:
1) Test the cut-off efficiency of recombinase CRE and VIKA;
2) Estimate the time needed for genome repair because mCherry can only be expressed after the cut off of SNAP UNIT and genome repair;
3) Fast cut off this part by transient transfecting recombinase gene into nucleus;
4) When there is no CRE, you can use this part as the SNAP UNIT, which can help localize proteins in vivo with BLOCK and dye from NEB company.
Source
The SNAP-tag® was brought from NEB company. NLS and sv40 terminator were got from commercial plasmid: pentry nls GFP/pcdna3.1. The sequence of Vox and DBOX was retrieved from Addgene and got through denovo synthesis. mCHERRY was got from commercial plasmid: pentry dcas9 mcherry bira. CYAN was got from commercial plasmid: puc18 cfp-yfp.
Construction Design
The SNAP-tag® and mCherry with tags and RTSs are constructed using PCR and oligonucleotide ligation by the following procedure. Our UNITs all contain XbaI and SpeI enzyme sites, so we can easily line up the UNITs using 3A assembly.
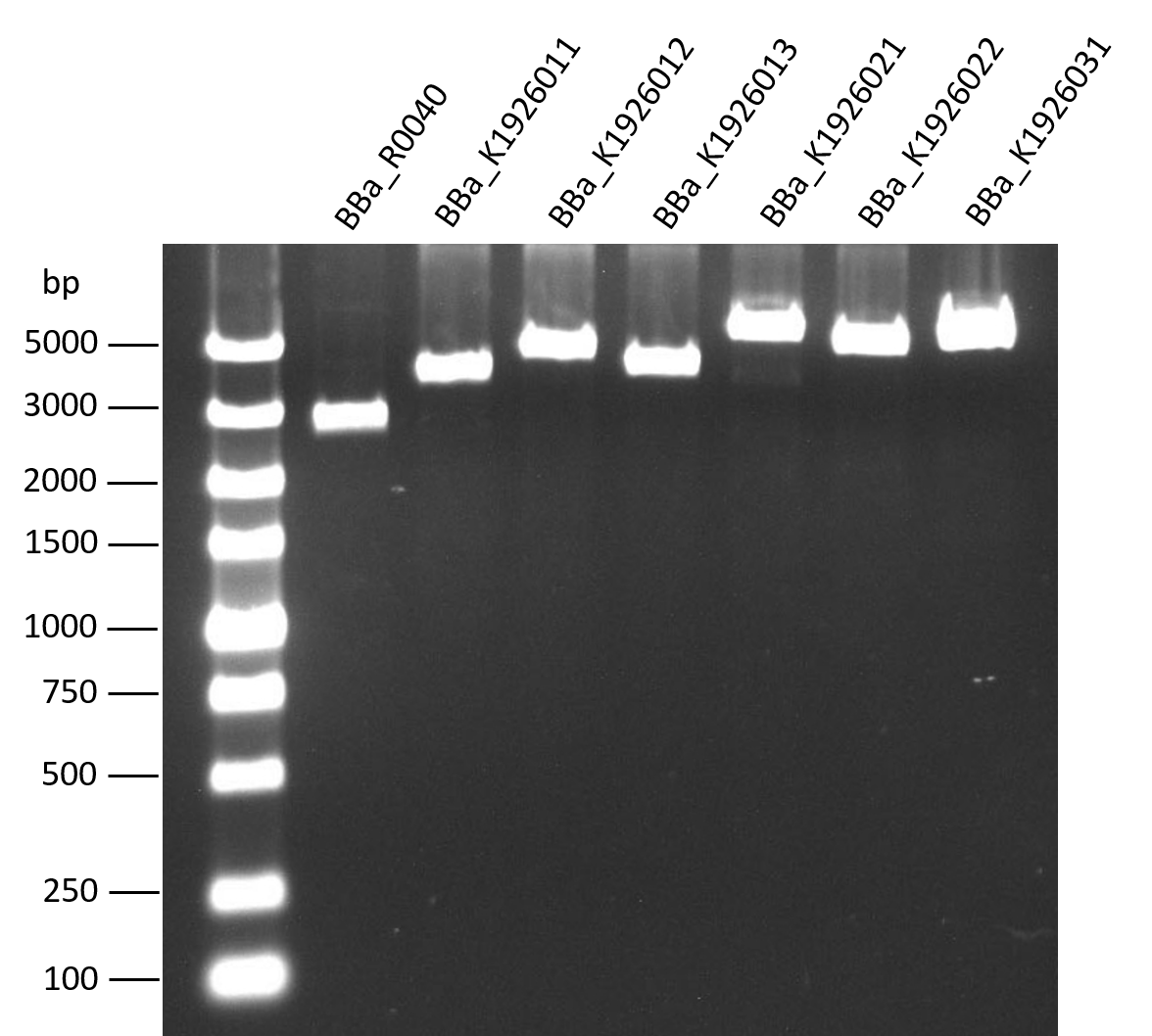
Gel analysis after EcoR1 digestion
The length of our biobricks are confirmed by gel analysis after EcoR1 digestion. The biobrick BBa_R0040, which is the negative control in 2016’s interlab, was used as a control because it only have a 54 bp part.
//chassis/eukaryote/human
| None |