Part:BBa_K808025
FsC: Cutinase PET cleaving enzyme
A cutinase is a cuticula degrading hydrolase from the fungus Fusarium solani pisi. It shows activity towards PET.
Usage and Biology
The characterization of FsC occured in 3 different ways.
Our [http://2012.igem.org/Team:TU_Darmstadt/Labjournal/Simulation Simulation Lab] performed
Our [http://2012.igem.org/Team:TU_Darmstadt/Project/Material_Science Material Science Lab] performed
Our [http://2012.igem.org/Team:TU_Darmstadt/Project/Degradation Degradation Lab]
Docking Simulation
[http://2012.igem.org/Team:TU_Darmstadt/Modeling_Docking#Docking Docking Simulations]
Here, OET (dimeric state) became our reference substrate, meaning if an additive exhibits a higher inhibition constant it may effect our protein. Otherwise, if a binding energy exhibits a higher level than OET we can conclude that an additive may accumulate on our protein surface. Moreover, we compare the binding as well as the inhibition energies of the additives with itself to identify the strongest binder. Since we know the active site of the Fs. Cutinase and the PnB Est. 13, it will be interesting if a plastizicer may dock there. In order to analyze that, we counted the residues which are involved within every docking simulation. Therefore, we measured the distance of every ligand to its corresponding receptor with an appropriate threshold and thus identified the residues.
For the sake of convenience of this page, please visit our [http://2012.igem.org/Team:TU_Darmstadt/Modeling_Docking#Docking Simulation Lab > Docking Simulation] for further informtion.
Surface analysis of polyethylene terephthalat with atomic force microscopy
[http://2012.igem.org/Team:TU_Darmstadt/Labjournal/Material_Science#Surface_analysis_of_polyethylene_terephthalate_with_atomic_force_microscopy_.28AFM.29 Surface analysis of polyethylene terephthalat with atomic force microscopy]
Atomic force microscopy is used to make very precise surface analysis up to nanometer scale. The goal was to indentify differences between different modifications of polyethylene terephthalate and to proof enzymatic degradation by changed surface properties.
Experiment 1
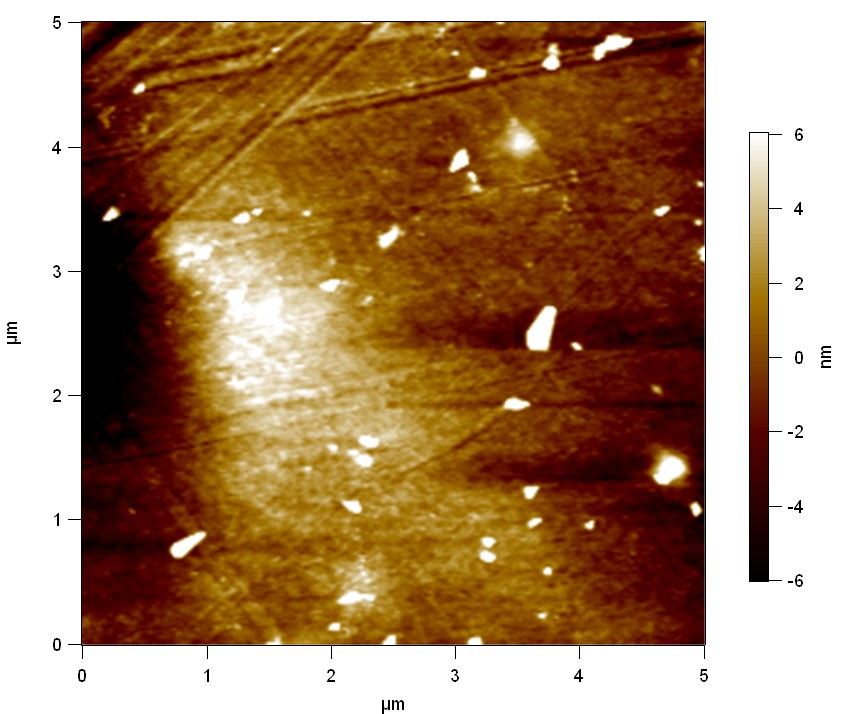
A piece of a PET water bottle is melted between two metal plates using a heat gun to create a flat sample. It is incubated for 24 h at 37 °C after applying a solution of 90 µmol/L FsC. The Sample is washed with distilled water and dried in a cabinet dryer at 60 °C for 1 h. The samples are investigated using AFM. Surface modifications of PET induced by FsC could be observed in form of circular perforations. Measurements in this area show a surface profile of (±100 nm). Measurements of the reference show a surface profile of (±30 nm). The pictures below show surface profiles and light microscopic captures of the non treated PET reference and the FsC treated PET surface.
AFM of PET surface after FsC exposure:
AFM of the PET surface as reference:
Experiment 2
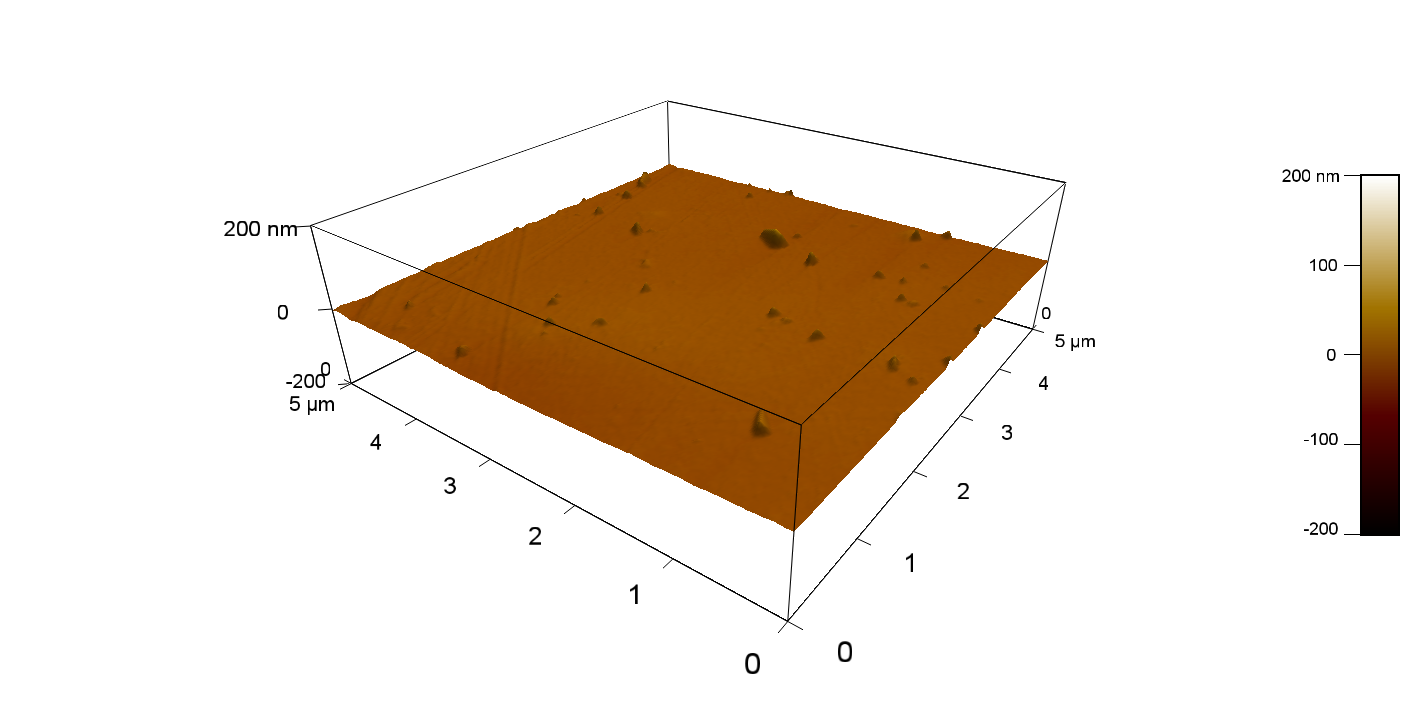
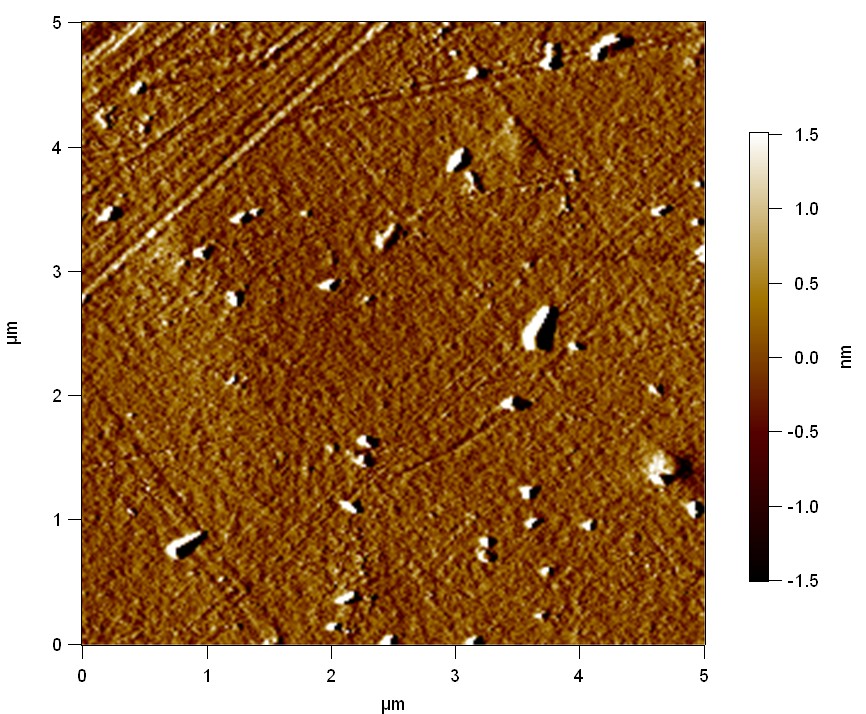
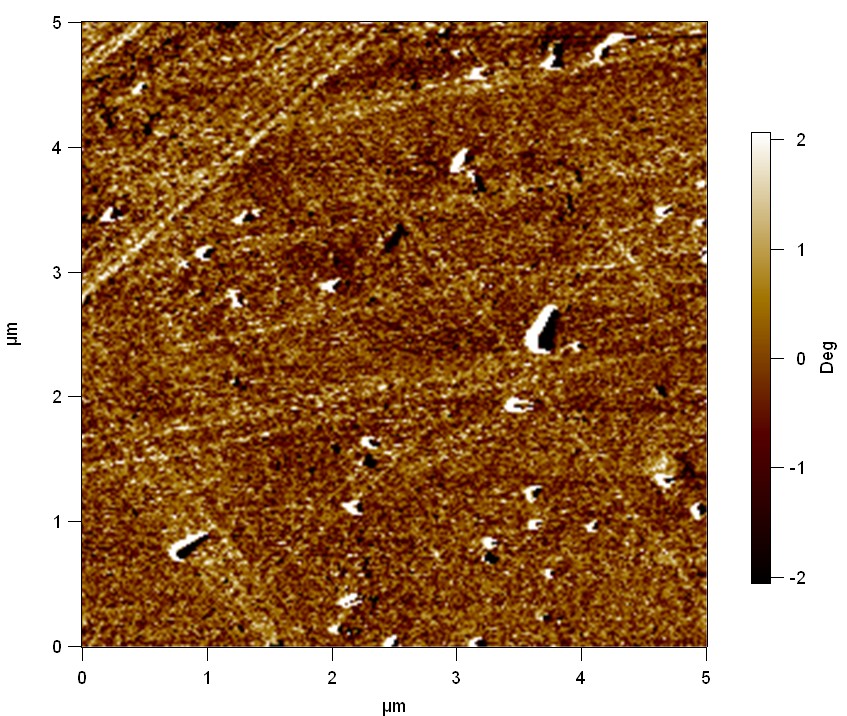
Pieces of PET foil were added to a 2 µmol/L solution of FsC. The solution was constantly agitated in 50 mL falcon tubes. After 7 days the samples were washed with distilled water and dried in a cabinet dryer at 60 °C over night. Afterwards the samples are investigated using AFM. FsC induced surface modification of PET could be observed. The Pictures below show 3D models, height profiles and the cantilevers amplitude as well as the phase deviations. The 3D models give a first impression of the texture of the surface. Looking at the height profile and the amplitude deviation more detailed informations about the surface texture can be obtained. The reference samples show a very flat surface (±3 nm). Bigger deviations are due to manufactionary mistakes of the foil. FsC treated surfaces show a deviation ten times bigger than non treated ones (±30 nm). Comparing the phase deviation of FsC treated surfaces with the reference, there can be seen big diferences due to varying mechanical properties. These are caused by degradation by FsC. The Polymer chains are cut in to smaller pieces which then stand out of the surface, letting it swell. The resulting surface is not only rougher but persumably less dense and therefore less firm.
AFM of PET foil after FsC exposure :
AFM of PET foil as reference :
Enzyme Activity Assay
[http://2012.igem.org/Team:TU_Darmstadt/Labjournal/Degradation#Enzyme_Activity_Assays Enzyme activity assay]
pNP-Assay
We used a [http://2012.igem.org/Team:TU_Darmstadt/Protocols/pNP_Assay#pNP-Assay pNP-Assay] in order to describe.
Conditions were set at: T = 34°C and pH = 7.4. The method is simple. The absorption is measured every minute over 30 minutes by an ELISA reader capable of 96 well plates, after addition of enzyme. The released pNP-group has a typical absorption at 405nm wavelength.
Km and Vmax calculation
To find Km the growth of the first twelve data points were written down in another diagram. The slope of the regression line gave the speed of the hydrolysis for the single substrate concentrations. Now the value of the speed with the associated concentration of substrate were plotted in a Lineweaver-Burk diagram. At the point where the regression line is crossing the x-axis the x-coordinate amounts to -1/Km. So Km is about 400µM. To calculate the value of Vmax we used the point where the plotted line crosses the y-axis. The associated y-coordinate is 1/Vmax. Consequently Vmax for 5nM FsC is 0,001467 1/s.
Kcat calculation
The Kcat value is calculated through dividing Vmax by the enzyme concentration of 5nM. Kcat counts 0,293395 1/mol*s.
Indicator Assay
For a more visual proof of enzymatic activity we used Monomethyl terephthalate as a substrate and FsC as a catalyst. We used [http://en.wikipedia.org/wiki/Bromothymol_blue bromthymol blue] as an indicator. The release of COOH groups, due to hydrolytic degradation by FsC results in a colometric change from green to blue. For a more detailed description please visit our [http://2012.igem.org/Team:TU_Darmstadt/Labjournal/Degradation#Degradation_of_monomethyl_terephthalate_over_time labjournal> Degradation of monomethyl terephthalate]
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NotI site found at 379
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 525
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 301
//function/degradation
| protein |