Part:BBa_K863101:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K863101
User Reviews
UNIQ12421c063a119b6b-partinfo-00000000-QINU UNIQ12421c063a119b6b-partinfo-00000001-QINU
|
•••••
Imperial College 2014 |
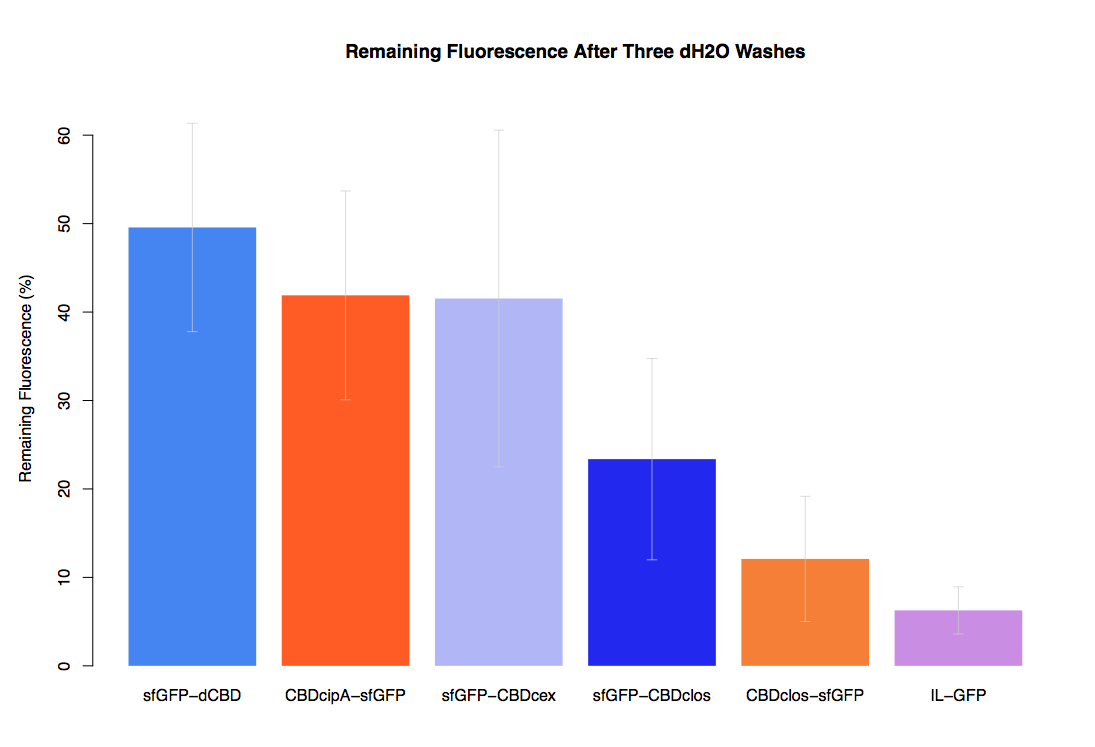
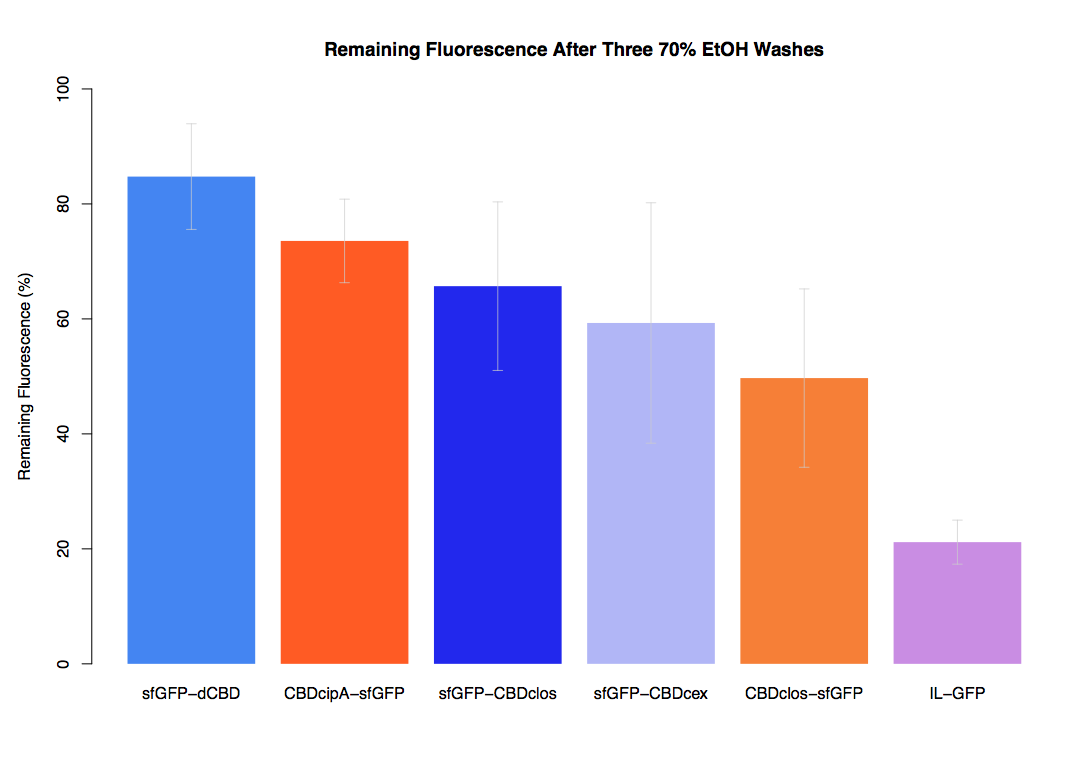
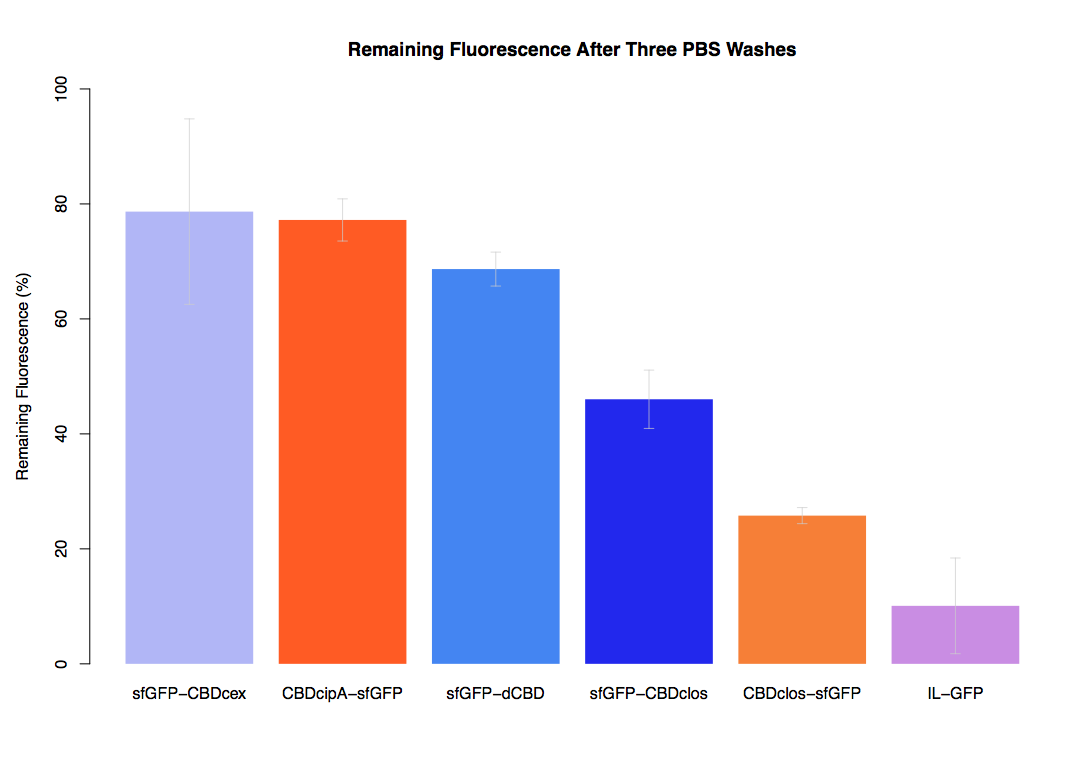
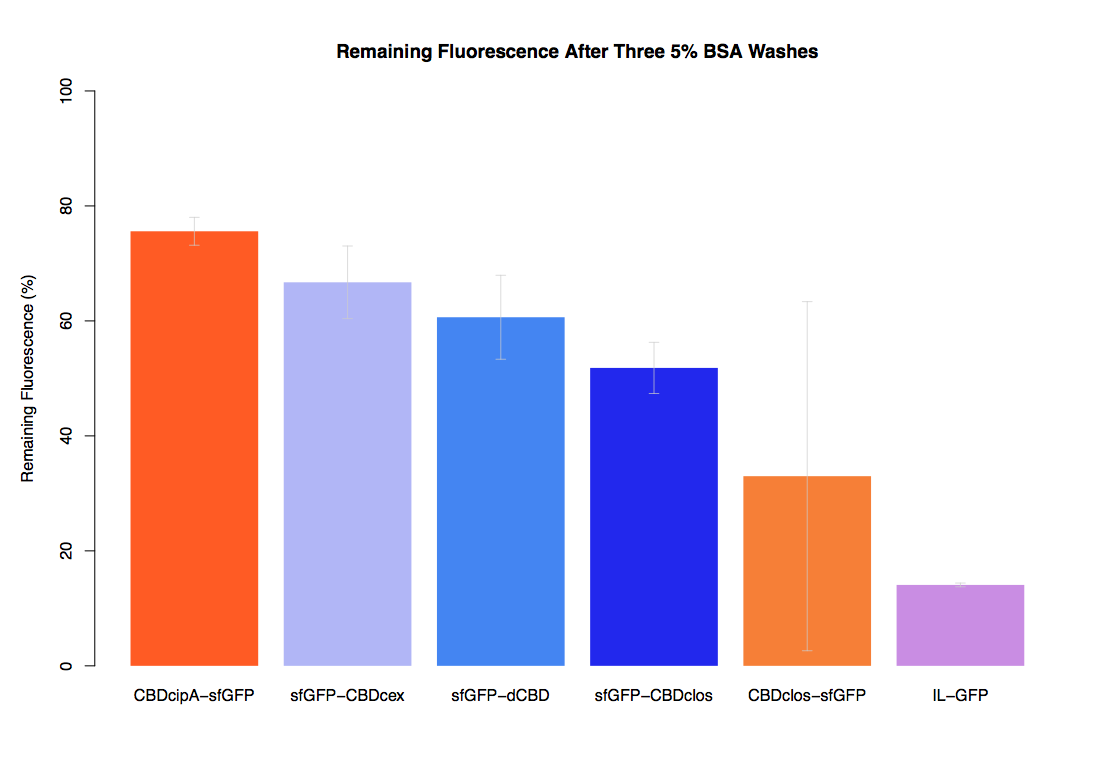
We successfully used this part: We fused it to sfGFP and expressed the fusion protein and characterised it's binding ability relative to other CBDs as described below: The binding ability of this CBD to bacterial cellulose was characterised when fused to sfGFP, relative to other CBDs fused to sfGFP. The binding ability was represented by the percentage fluorescence remaining from the sfGFP-CBD fusions bound to bacterial cellulose discs, when subjected to various washes (protocol [http://2014.igem.org/Team:Imperial/Protocols here]). When washed with PBS, sfGFP-CBDcex fusions performed the best out of all the CBDs tested (third graph) followed by washes with 5% BSA (fourth graph), dH20 (first graph) and 70% EtOH (second graph). .
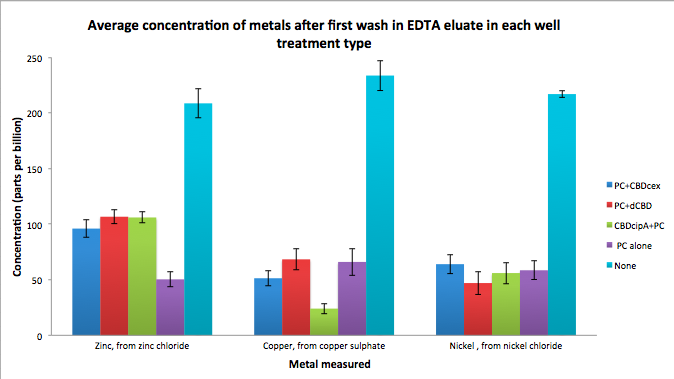
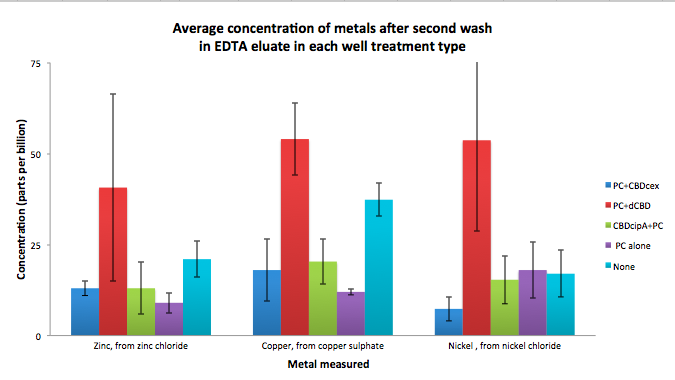
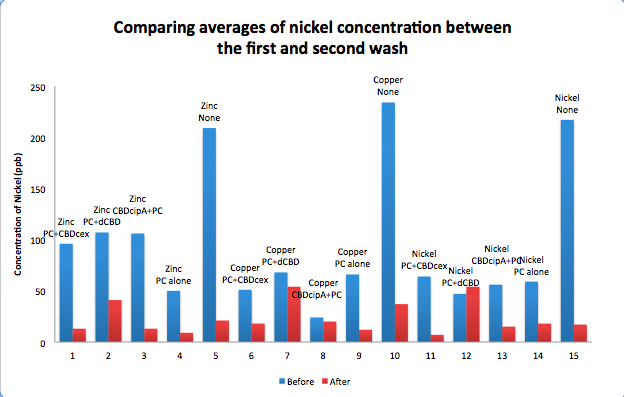
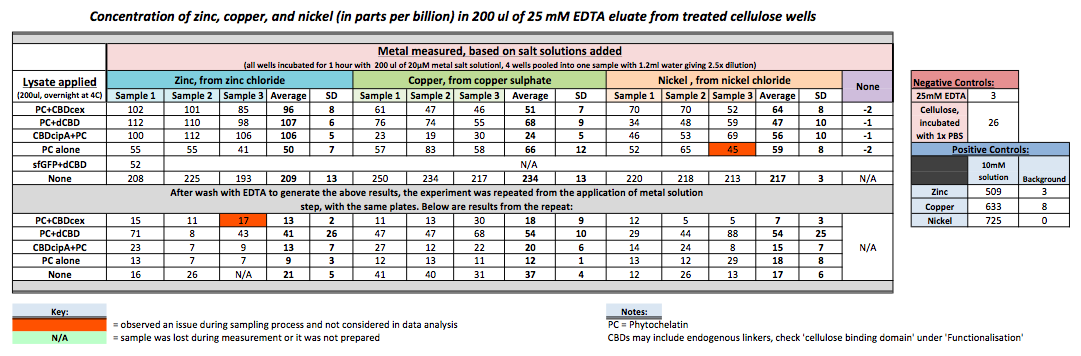
Phytochelatin fused to our CBDs were bound onto cellulose that were dried in the bottom of 96-well plates and tested against 3 different metals (nickel, copper, zinc). First, the fusion protein cell lysate was incubated overnight in the cellulose wells. Following this, the metal salt solutions are added in excess into the wells. Finally, an EDTA step removes the bound metal ions into solution, and the metal concentration in solution is quantified by mass spectrometer. Multiple washes with PBS and water were done between each binding step, ensuring that the metal ions that are measured were released from the phytochelatin. To evaluate if these CBD fusions were reusable, we re-applied metal ion solutions onto the same wells with the CBD fusions adhered. We washed the wells as before, then eluted with EDTA in the same manner. The results from the each elutions are shown below, with the final graph comparing between the first and second wash. We found along the way our bacterial cellulose alone had some metal chelating properties, as was also confirmed by our filtering set up with the dCBD-phytochelatin, but the second elution seems to show less background noise as the EDTA disrupts cellulose's natural binding to the metal ions. The full table of results are shown below as well. The full assay protocol can be found as ‚ÄėMetal binding assay protocol‚Äô at this link: http://2014.igem.org/Team:Imperial/Protocols
|