Part:BBa_K1022102
pT7: RBS: His6- SUMO: Signiferin
This part codes for the peptide 'Signiferin' tagged with 'His-6-SUMO' molecule. Its production is triggered by IPTG (through pT7 promoter).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 46
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 170
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 355
- 1000COMPATIBLE WITH RFC[1000]
Characterization
For more info, visit [http://2013.igem.org/Team:TU-Delft/Peptides#SUMO TU Delft iGEM13 Wiki]
Peptide production
Introduction:
In the project by iGEM 13 Team TU Delft, a SUMO-peptide fusion was opted as a suitable expression system as they make the fusion proteins more soluble. The peptide by itself is not soluble in the cytoplasm but making a fusion of peptide with Small Ubiquitin like Modifiers (SUMO) will increase the solubility of the peptide, thus increasing the cytoplasmic fraction of the peptide.
A gene was constructed in such a way that the SUMO-peptide production was driven by the strong T7 phage promoter. This gene containing plasmid was harbored in a BL21(DE3) strain that has lac promoter driven T7 polymerase. Upon induction by IPTG the SUMO peptide fusion is produced as a soluble protein fraction.
The protocol can be seen [http://2013.igem.org/Team:TU-Delft/Protocol_10#protocol_10 here].
Result:
Discussion:
Peptide Characterization (MIC)
Introduction:
An important part of the project is inhibition of growth or killing of bacteria with the use of antimicrobial peptides (AMPs). In order to get an idea of the toxicity of our peptides we conducted several minimal inhibiting concentration (MIC) experiments with synthetic peptides ordered from DNA 2.0. These MIC measurements where done on E. coli, B. subtilis and S. delphini, with the first as representative of our Gram-negative expression host, the second for the Gram-positive targets and the last for our specific target.
MIC determination:
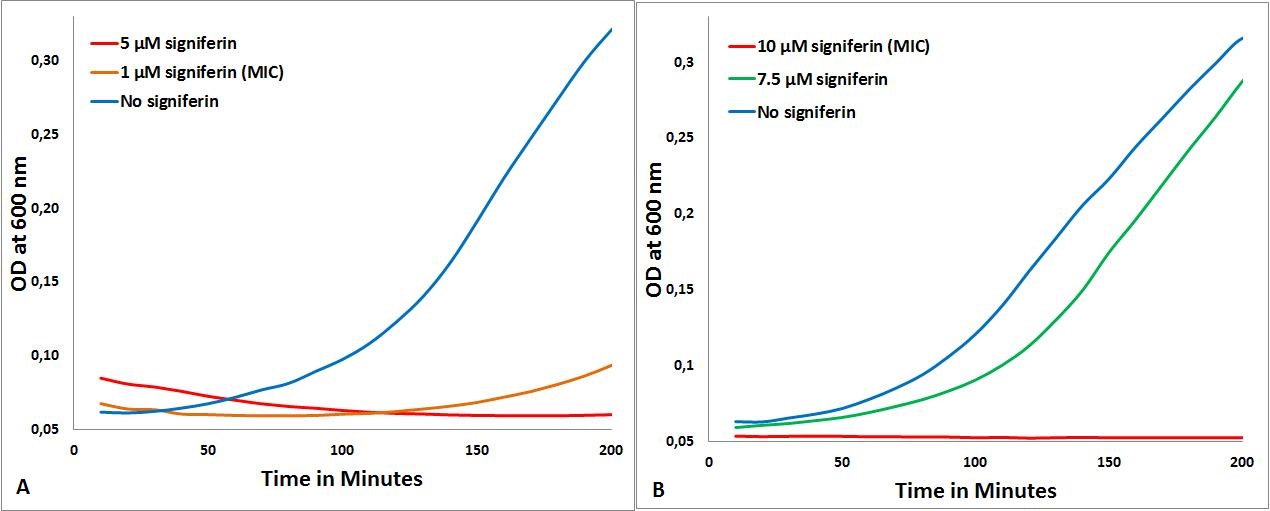
The MIC of signiferin on S. delphini, B. subtilis and E. coli were done according to the protocol described [http://2013.igem.org/Team:TU-Delft/PeptideCharacterization here]. S. delphini is the most sensitive for signiferin, as the MIC was determined to be 1µM (part A in figure below), no growth is seen at this concentration. The MIC for B. subtilis was determined to be 10µM (part B in figure below).
- MICs of signiferin. 1A: signiferin on S. delphini, 1B: signiferin on B. subtilis
The expected selectivity of the chosen peptides for Staphylococcus was confirmed by these experiments, as for all the peptides for which we could determine a MIC, that MIC was the lowest on S. delphini.
For MIC by other peptides used in iGEM 2013 Team TU Delft, Click [http://2013.igem.org/Team:TU-Delft/PeptideCharacterization here].
Comparison with two peptides:
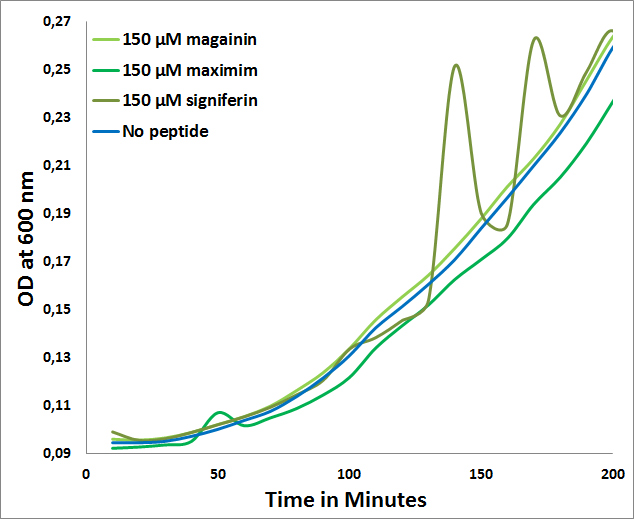
The fact that for none of the peptides a MIC could be determined for E. coli (>150µM) further confirms the expected selectivity towards Gram-positives which is observed in the following graph:
- MICs of Maximim-H5, Signiferin and Magainin on E. coli
COS-1 toxicity determination:
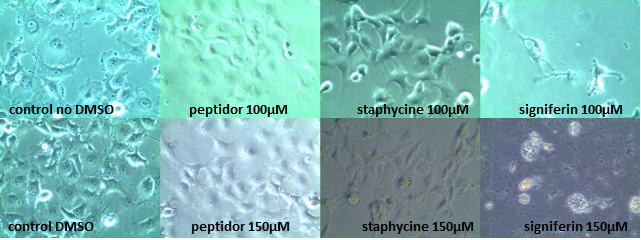
In order to determine the toxicity of the AMPs on mammalian cells we tested the most promising peptides on COS-1 cells. As signiferin, staphycine and peptidor gave the best results in the experiments of MIC, they were chosen to be tested with concentrations up to 150µM on COS-1 cells. COS-1 cells were chosen as they, because of their simian nature, strongly resemble human cells with respect to membrane properties and crucial cell functions and mechanisms. Normally healthy COS-1 cells attach to the bottom of the well, showing a clear fibroblastic morphology. When they start to die they will swell and eventually detach from the bottom forming spherical cells which upon lysis leave behind cell debris.
Signiferin showed to be toxic above 100µM, whereas staphycine and peptidor turned out not to be toxic up to concentrations of 150µM. Two controls are shown, as peptidor and signiferin are dissolved in water whereas staphycine is in 80% DMSO. All photos shown are taken 24 hours after induction, but are representative for the photos taken 4 hours after induction. In the 150µM signiferin experiment no live cells could be observed at both 4 hours and 24 hours after induction.
For more info, visit [http://2013.igem.org/Team:TU-Delft/PeptideCharacterization TU Delft iGEM13 Wiki]
| n/a | pT7: RBS: His6- SUMO: Signiferin |