Part:BBa_K1022100
pBAD:AIP receiver:GFP:TT
This plasmid contains code for pBAD promoter, AIP receiver Agr A and Agr C, GFP along with double terminator.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 125
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 521
Illegal BamHI site found at 65
Illegal BamHI site found at 1405 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 2970
Characterization
For more info, visit [http://2013.igem.org/Team:TU-Delft/Killswitch TU Delft iGEM13 Wiki]
Sensor Experiment
Introduction:
In this sensor test-construct AgrC and AgrA are expressed, after which AgrC can act as a receptor for AIP. After binding AIP, AgrC will phosphorylate AgrA which then starts acting as a postitive transcription factor for pP2. Therefore, upon induction by AIP, the construct will give a GFP signal. This is tested using the following experiment.
Experimental Set Up:
Cells are induced with 0.1 % Arabinose (to induce pBAD) and AIP’s, and with only AIP. Induction with AIP is done at 1µM and 10 µM. 3 hours after induction fluorescence-activated cell sorting (FACS) is used to check for GFP signals. The controls are BL21(DE3) cells, uninduced plasmid containing BL21(DE3) and a constitutively expressed GFP construct containing strain. The AIP used to induce the cells was synthesized by Eurogentec.
The cells induced with arabinose all died, which could be seen by the fact the signal given by the FACS, this could be due the fact AgrA and/or AgrC are toxic in high concentrations. The fact E. coli is still able to detect AIPs without induction of the Agr genes is explainable due the fact pBAD is a bit leaky, making low transcription possible, apparently only a small amount of activated AgrA is required to give a measurable level of GFP.
Earlier work on the detection of AIPs by Gram-negatives has been done, but with unclear or non-convincing results. Often the outer-membrane of E. coli was seen as an obstacle for the implementation of auto-inducing peptide sensing systems in Gram negatives. Although, after intensive literature research we found a molecule very similar to the AIP molecule used in these experiments, that is known to pass the outer membrane. The molecule bacitracin has been shown to interfere with cell wall synthesis in Gram negative species, therefore being able to pass the outer membrane as cell wall synthesis occurs in the periplasm. Comparison of the molecular structures of bacitracin and AIP regarding size, molecular weight, polarity and charge gave us an indication AIP should be possible to pass through the outer membrane of E. coli.
Results:
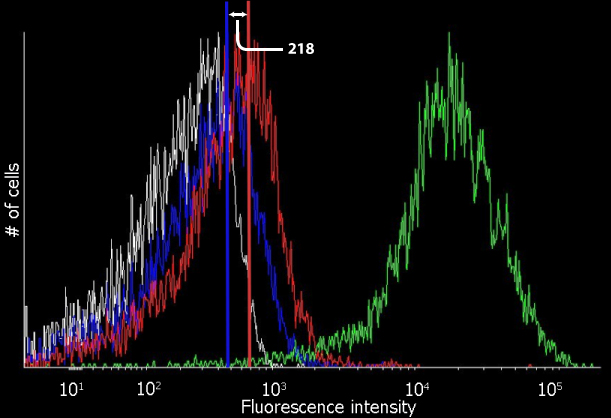
Using Cyflogic 1.2.1 software, FACS data was analysed, showing a clear induction of the AIP receiver GFP construct by AIP. The difference between the blue and the red line (218) indicates the difference in fluorescent signal of uninduced and induced cells. Untransformed Bl21(DE3) and constitutively expressed GFP were used as respectively negative and positive control. The significant difference between the uninduced and the induced samples is determined with a P-value >10-4 as described in the next paragraph.
Fluorescent signal measured with the FACS. The white line refers to the control BL21(DE3) cells. In blue the uninduced pBAD AIP receiver GFP is shown. The red line represents the AIP induced receiver cells. The fluorescence intensity scale is logarithmic and the total number of cells counted per sample is 10,000.
Significance test:
To test if the difference between the uninduced and induced result is significant a statistic test is performed as in. Here it is assumed that both results are normal distributions, as the number of data sets is large (central limit theorem). Then the critical value equation becomes a simple one:
Based on the induced and uninduced data as in Table below, this t-value is calculated as 52.7. This is a very high value and corresponds to a one-sided p value of less than 10-4. This means that there is a significant difference between the induced and uninduced curves.
| Dataset | Mean (μ) | Standard deviation (σ) | Number of samples (N) | |
|---|---|---|---|---|
| Non-induced | 465 | 242.6 | 10.000 |
//function/reporter/fluorescence
receiver
| biology | E.coli |
| emission | Green |
| excitation | |
| function | Senses the Auto inducing peptides and emits Green fluroscence |
| n/a | pBAD:AIP receiver:GFP:TT |
| tag | None |