Part:BBa_K801020
KlADH4 yeast promoter, ethanol inducible
This part is the ethanol inducible promoter controlling the KlADH4-gene of K. lactis.
The use of this ethanol inducible promoter to produce heterologous proteins in K. lactis was shown by Salioa et al. 1999 [http://www.ncbi.nlm.nih.gov/pubmed?term=9872759].
We characterized this part in S. cerevisiae (strain INVSc1) to find out whether this part is also ethanol-inducible in this yeast.
Our results are still ambigous, but at this point our data suggests that the part is also ethnaol-inducible in S. cerevisiae . Further experiments will be performed to clarify this.
Usage and Biology
The UASe-region of this promoter has been shown to be responsible for the ethanol sensitivity of this promoter in K. lactis (Mazzoni et al., 2000 [http://www.ncbi.nlm.nih.gov/pubmed?term=10724480]). The region includes binding sites for the Rap1-protein (repressor activator protein 1) and the Yap1-protein (a transcription factor involved in stress response) as well as two heat shock elements (HSE) and five stress response elemtents (STRE). All these cis-elements and the respecitve proteins also occur in S. cerevisiae. For this reason we wanted to examine whether the KlADH4-promoter remains ethanol inducible if it is transferred from its natural organism (K. lactis) to S. cerevisiae.
The characterization of this part was done using a KlADH4-promoter + eGFP construct.
First experiment using over-night pre-cultures
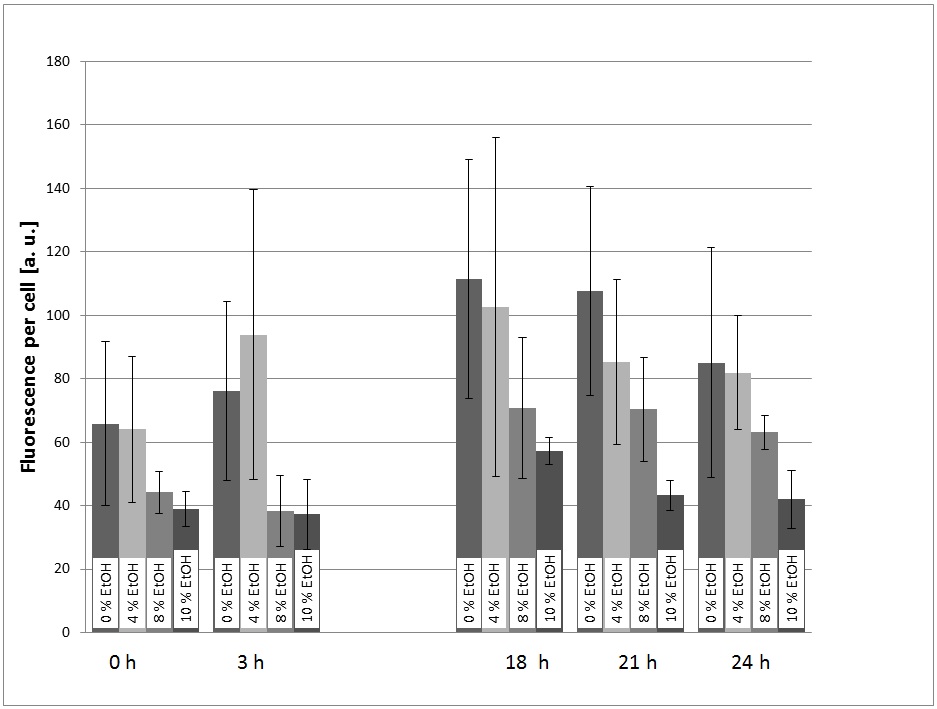
In a first experiment, the transformed yeast cells were picked grown in a pre-culture (SC-U Medium, 30 °C, 180 rpm) over night and transferred into SC-U Medium with different concentrations of ethanol (0%, 4%, 8%, 10%, v/v). The eGFP-fluorescence and the OD600 were measured at t = 0h, 3h, 18h, 21h, 24h. Also, the ethanol concentration of the cultures was measured using an Alchohol-Dehydrogenase-Assay.
For the evaluation of the experimental data, the measured fluorescence was divided by the respecitve OD600, to normalize the fluorescence to the respecitve cell count. This was done to take the intrinsic auto-fluorescence in account. The results are shown in picture 1.
The promoter is generally functional in S. cerevisiae, which can be seen by the fact that eGFP is expressed (also see picture X).
The fact that the expression of eGFP is low in the cultures with 8% and 10% (v/v) ethanol can be explained with the fact that the viability of the yeast cells is dramatically decreased at these high ethanol concentrations. This can also be seen by the growth curves (picture 2).
At first glance, the fact that there is a significant signal in the culture with 0% ethanol added looks as if the promoter is constitutive and not specifically induced by ethanol. However, the cells could also be induced by the ethanol produced by the yeast themselves during the over night pre-culture. Because of this ambiguity, further experiments were performed.
Second experiment: Characterization of the KlADH4-promoter without over-night pre-cultures
The goal of this series of experiments was to avoid pre-cultures in which the cells would produce alcohol and hence be induced before the actual measuring series. Therefore, three measures were taken:
- Use of baffled flasks to increas oxygen transfer into the medium, thus lowering fermentation
- Transfer of a large amount of transformed yeast cells into SC-U Medium with different ethanol concentrations immediately after transformation and start measuring the eGFP-expression. The transformed cells were not plated on selective plates and thus, picking of clones and a pre-culture became unneccesary. (Experiment 2.1)
- Cultivate a control culture of transformed yeast in SC-U Medium with Glycerol as sole carbon source. Glycerol is a non-fermentable carbon source for S. cerevisiae (Feldmann, 2005). (Experiment 2.2)
Result of experiment 2.1: cells transferred into liquid medium immediately after transformation
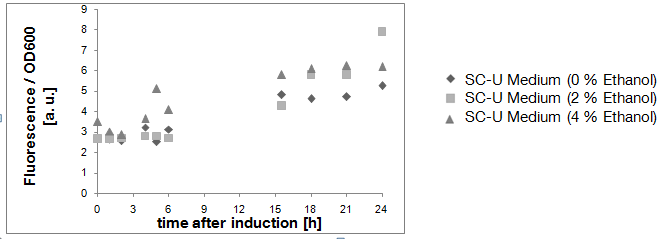
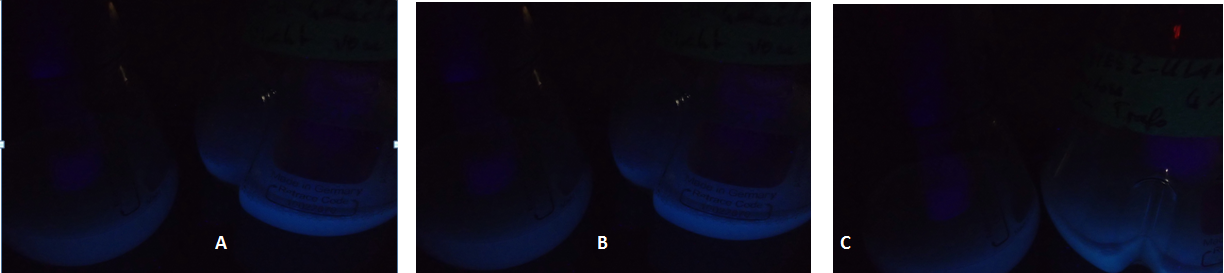
Due to the fact that the cultures examined here also contain untransformed yeast cells, the OD600-value is falsely high. The untransformed yeast cells are not viable (due to uracil auxotrophy and cultivation in SC-U, a medium lacking uracil) and cannot express eGFP, but still they disperse light and thus increase the measured OD. This renders the data shown in Fig. 3 uninterpretable. However, the cultures which were used for these measurements expressed eGFP - enough to produce fluorescence visible to the naked eye (Fig. 4).
Result of experiment 2.2: negative culture grown in SC-U glycerol
The aim of this experiment was to keep the ethanol production as low as possible by the use of glycerol as sole carbon source. As shown in Fig. 5, the Ethanol concentration in the culture remained very low (less that 0.21 Vol.-%). Also, the "Fluorescence per cell" does not increase over time - the yeast cells do not express eGFP.
To verify the fact that the cells did not express eGFP, excitation and emission spectra were recorded at t = 24 h. For comparison, reference spectra
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 753
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 590
References
- [1] Saliola, M., Mazzoni, C., Solimando, N., Crisà, A., Falcone, C. & Jung, G. (1999) ‚Use of the KlADH4 promoter for ethanol-dependent production of recombinant human serum albumin in Kluyveromyces lactis’, Appl Environ Microbiol. 65 (1), 53-60. [http://www.ncbi.nlm.nih.gov/pubmed?term=10724480 PMID: 9872759]
- [2] Mazzoni, C., Santori, F., Saliola, M. & Falcone, C. (2000) ‚Molecular analysis of UASE, a cis element containing stress response elements responsible for ethanol induction of the KlADH4 gene of Kluyveromyces lactis’, Res. Microbiol. 151, 19-28. [http://www.ncbi.nlm.nih.gov/pubmed?term=10724480 PMID: 10724480]
//promoter
| None |