Part:BBa_K731020
Inducible araC-pBAD promoter regulating WT CysE
The CysE gene (K731000) is here regulated by the araC-pBAD promoter (K731201), which is inducible by arabinose.
This part has been successfully operated both in pSB1C3 (K731020) and the low copy vector pSB3C5, in which it was characterized.
A mutated version of this construct is available as Part BBa_K731030, which is said to have greater cysteine production and secretion, being it insensitive to feedback inhibition by cysteine.
This part was cloned by the iGEM Trento 2012 team for the creation of an aerobically engineered pathway for the removal of the black crust disfiguring marble stones. Further information about this part and its characterization can be found in the iGEM Trento 2012 wiki.
This Part is also available into the medium copy vector pSB3C5. They are available upon request ( igemtrento [at] gmail [dot] com )
Usage and Biology
In cysteine biosynthesis, CysE (a serine acetyltransferase) catalyzes the acetylation of serine to give O-acetylserine, a precursor for the biosynthesis of cysteine. Some O-acetylserine is also converted to N-acetylserine, which in turn triggers the assimilation of sulfate through sulfate assimilation genes.
In Escherichia coli conversion of L-serine to L-cysteine is mediated by the action of two enzymes: serine acetyltransferase [1] catalyses the activation of L-serine by acetyl-CoA. Its product, 0-acetyl-L-serine (OAS), is then subsequently converted to L-cysteine by 0-acetyl-L-serine(thio1)lyase.
The synthesis of OAS-(thio1)-lyase and of the enzymes involved in sulphate uptake and reduction is regulated by induction as well as by repression [2].
The expression of cysE (the SAT structural gene), on the other hand, is constitutive whereas the catalytic activity of the gene product, SAT, is sensitive to feedback inhibition by L-cysteine [3].
The pBAD promoter is active in presence of L-arabinose. L-arabinose binding to the AraC protein inactivates the AraC inhibitory function, permitting RNA polymerase to start transcription on cysE, which controlled by pBAD.
AraC is also negatively regulated by cAMP via CRP (formerly known as CAP, catabolite activating protein). In presence of glucose, cAMP levels are low, meaning that AraC can still act as a repressor, not allowing transcription. In the original ara operon, this circuit has the function to limit its transcription when arabinose in not needed, which is when it’s not present and/or when glucose (the primary energy source) is available.
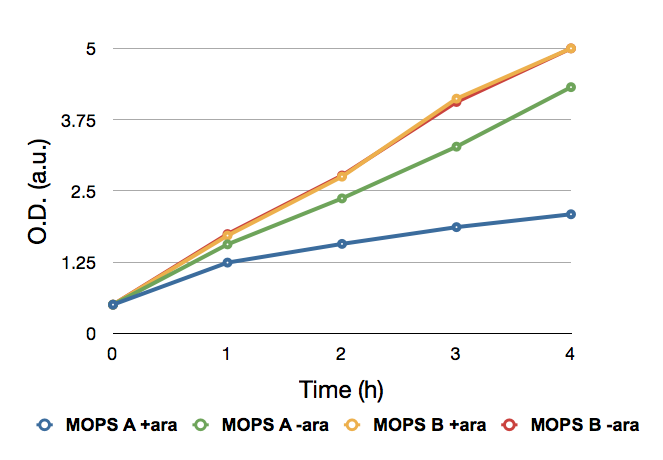
FIGURE 1 Effect of CysE in cell growth. Cell density was measured at different time points to determine the effect of CysE expression. Cells were grown at 37°C in LB until it was reached an OD of 0.6. The cells were then spinned down and resuspended in an equal volume of MOPS medium and allowed to grow to an OD of 0.8. Prior the induction cells were splitted into two samples of equal volume. One of the two samples was induced with 5 mM arabinose. Every hour a 0.75mL volume was taken to measure the OD. This assay was performed in two different MOPS media: with 60 mM glycerol (MOPS A) and with 30 mM glucose (MOPS B).
When grown in glucose (MOPS B), cells show an higher O.D. than those grown in glycerol (MOPS A). When induced, cells grow at lower O.D. than the corresponding not induced culture. The pBAD promoter is active in presence of L-arabinose. L-arabinose binding to the AraC protein inactivates the AraC inhibitory function, permitting RNA polymerase to start transcription on cysE, which is controlled by pBAD. AraC is also negatively regulated by cAMP via CRP (formerly known as CAP, catabolite activating protein). In presence of glucose, cAMP levels are low, meaning that AraC can still act as a repressor, not allowing transcription. In the original ara operon, this circuit has the function to limit its transcription when no arabinose is present and/or when glucose (the primary energy source) is available. When grown in MOPS B cells can use glucose. cysE, which is regulated by araC-pBAD, should not be transcribed, whereas in MOPS A (containing glycerol and no glucose as the carbon source), the protein should be expressed.
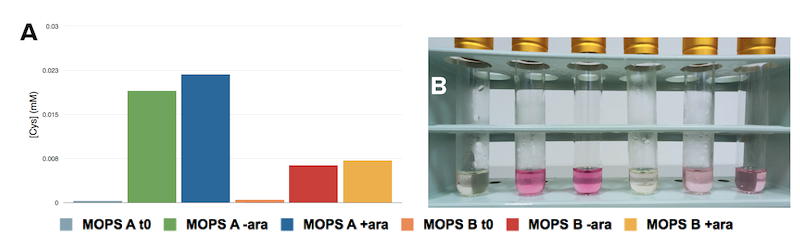
FIGURE 2 Cysteine production upon arabinose induction. Cells were grown as described in Figure 1 and left to grow overnight. A modified version on the assay proposed by Gaitonde et al. was adopted to measure cysteine production in cultures. The reagent was prepared mixing 250mg of ninhydrin in 10mL of a solution made of glacial acetic acid (60%) and fuming HCl (40%). Solubilization of ninhyrin occured after about 15min of vortexing at max speed. Samples were prepared in glass tubes, mixing 0.5mL of glacial acetic acid, 0.5mL of the culture to be tested and 0.5mL of the reagent previously described. The mixture was left 10min in a 90°C water bath. In presence of Cysteine, ninhydrin makes the solution turn pink/purple in about 3min. Spectra were recorded from 600nm to 400nm, as the characteristic intensity peak for cysteine is at 560nm. Cysteine concentration was calculated referring to a standard curve.From left to right cells expressing K731020 in: MOPSA before induction, MOPS A- arabinose after 16h, MOPS A + arabinose after 16h, MOPS B before induction, MOPS B - arabinose after 16h, MOPS B + arabinose after 16h.
Serial dilutions data is confirmative of conclusions coming from growth curves. When grown in MOPS A cultures show a marked decrease in their number. Induction, similarly to what was seen in growth curves, lowers the cell number.
Head over to K731030 for a comparison between the WT and M256I version.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 1144
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 979
Illegal AgeI site found at 1909 - 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI site found at 961
| None |