Part:BBa_K4439007
mSA-N[AS]4C-CBD-10xHis
Contents
Abstract
To complete
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Protein Characterization
Usage and Biology
- Silk proteins demonstrate interesting mechanical properties such as toughness, strength, lightweight, biodegradability and the possibility to produce different morphologies (fibers, foams, capsules, films). In addition to this, silk proteins comprise a high percentage of the amino acids glycine, serine and alanine which have an intermediate hydrophobicity.
- Green lacewing insects produce two types of silk: one produced by the larvae (cocoon) and the other by adult females (egg-stalk). The adult produced silk acts as a protective shelter and structural support for egg stalks, which are two ideal properties for a waterproof coating for our aerogel.
- In green lacewings, two serine- and glycine-rich silk proteins (Ma1XB1 and Ma1XB2) have been identified, both with highly repetitive core domains and small terminal domains. The core domain’s structure is rich in β-sheets with an approximative sheet-length of four amino acids between turns. These form repeating structural units constituting β-helices which have a significant positive correlation with the proteins’ surface hydrophobicity. A consensus motif for the core domain of Ma1XB2 (named [AS])had already been generated. Furthermore, a recombinant protein constituted by 8 repetitions of this [AS] module had also already been expressed in E. coli.
- We decided to use the N[AS]4C protein in our plasmid construct in the end because the gene synthesis of 8 repetitive elements is quite difficult to perform. Also, this allowed us to have a smaller protein in the end, which leads to less risks of problems in the expression.
Modeling

Figure 8 | AlphaFold2 prediction for 01a. (A) First rank 3D model prediction of 01a protein; the iteration who got the highest score in the modeling. (B) Different correlation graphs between the query sequence of 01a and the predicted one, for each proposed 5 models. (C) Figure of sequence coverage of 01a and indices on alignment with other sequences in the mSA. (D) IDDT graph for 01a per residue to get an idea of the confidence of the model in predicting the geometry.
- Analysis : According to (fig. 8, A), we could identify the structure of the different single chains of interest easily. The blue slightly smaller helicoidal structure represents the mSA protein that would be attached to the silk protein, the elongated green structure and finally we would get the CBD sequence that is displayed in red. The (fig.8, B) confirms the hypothesis of knowing very efficiently the three domains and having trouble distinguishing the in-between linkage which is totally normal since we designed those linking segments to be able to keep the structure of the three main elements. The same graph showed that few iterations of the prediction lacked to characterize the silk segment, which could be an issue in the experiments. However both (fig. 8, C) and (fig. 8, D), confirmed the reasoning that our proteins would keep a structure preserving their initial aim. The linkage would give in reality more freedom of placement of those chains. The IDDT score helped to identify the percentage of correctly predicted and true structure that could be superimposed. In general, the higher this score the better the model is considered.
Experiments
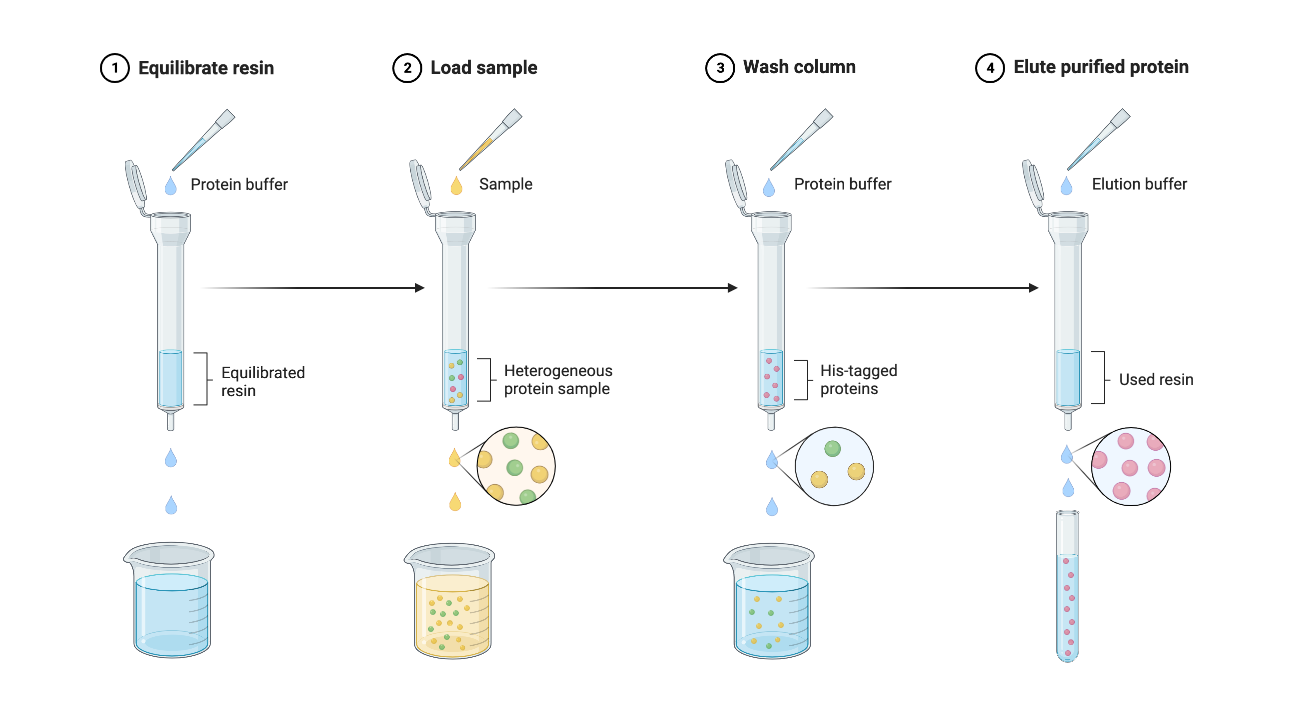
Figure 1 | Protein Purification Protocol using Ni-NTA Beads
- For more information, check out our Protocols page on our wiki.

Figure 2 | Diagram of Procedure for Silk Biofilm Fabrication
- For more information, check out our Protocols page on our wiki.
Lab's Tips and Tricks
- Purification might prove difficult : elution with EDTA works better but might damage the protein more.
- The silk biofilm can be created even if the recombinant protein is in liquid form.
Results
Bacterial Transformation
We followed the E.Coli competent cells quick protocol FB035 of Promega. We transformed BL21(DE3) cells with our plasmid containing mSA-N[AS]4C-CBD-10xHis. Cells were incubated on ice for about 10 min before heat shock. 400 µl of SOC medium was added after the heat shock. 100 µl of transformation reaction was plated on LB-Kana plates and the 300 µl left were put in 50 ml of LB-Kana in an Erlenmeyer to do liquid transformation cultures overnight at 37°C under shaking (200rpm).

Figure 3 | Bacterial transformation with the 01a construct in BL21(DE3) competent cells. (A) The plate transformed with 01a. (B) The plate transformed with a plasmid containing standard GFP (positive control). (C) The plate transformed with water (negative control).
After the overnight incubation, we observed the presence of colonies in the BL21(DE3) transformed cells plates of 01a and the positive control, no colonies were observed on the negative control, as expected.
. OD600 measurement: 2.914 was reached after the overnight incubation of the liquid transformation culture.
- Analysis
The 01a GeneScript plasmid transformation worked well for BL21(DE3) since we can observe colonies on the LB-Kanamycin plates and the OD600 of the transformation liquid culture is high, meaning that many bacteria were able to grow.
Purification
The purification was performed following a Ni-NTA beads based protocol.

Figure 3. Protein purification of silk fusion protein (01a)(A) SDS-PAGE gel stained with Coomassie Blue Protein Stain of all the fractions of silk fusion protein purification (B) Western Blot of the elution 1 (E1 fraction on SDS-PAGE gel) visualised with anti-His antibody. W1 = Wash 1 with 20 mM imidazole; W2 = Wash 2 with 50 mM imidazole; E1 = Elution 1 with 250 mM imidazole; E2 = Elution 2 with 500 mM imidazole; E4 = 2.5 M imidazole; E5 = 5 M imidazole.
- Analysis
Protein purification of the mSA-N[AS]4C-CBD-10xHis protein (01a) showed a 100kDa protein present in the first elution lane (E1) in both the SDS-PAGE (fig 3.A) and the Western Blot (fig 3.B). mSA-N[AS]4C-CBD-10xHis protein was expected at 78kDa size. Using the Nanodrop, we measured the concentration (0.72 mg/mL) and we obtained a final amount of 2.15 mg of the mSA-N[AS]4C-CBD-10xHis protein (01a). Since high imidazole concentration might denature the proteins, we had to either remove it or make imidazole inert. It was not possible to dialyse the proteins to remove imidazole, since the dialysis membrane was made of cellulose, and our fusion proteins would all bind to the membrane via their cellulose binding domain (CBD). We therefore decided to flash freeze the proteins and store them in the elution buffers so the imidazole will no longer affect them when frozen.
Biofilm Fabrication

Figure 9 | Silk biofilms inside polystyrene petri dishes. (A) Silk fusion protein biofilm in polystyrene petri dish after 24 hours drying. (B) GFP fusion protein biofilm in polystyrene petri dish after 24 hours drying.
- Analysis
After the production of our proteins, we created a silk fusion protein biofilm to coat an aerogel to make it water-resistant. Inspired by the work of Felix Bauer, we produced two biofilms: one with the recombinant silk protein (fig 9.A) and one with the recombinant GFP protein, as a control (fig 9.B). In figure 9, we can see that we dried the biofilms too much due to the presence of cracks. Even if it was not a problem for hydrophobicity testing, it became one to remove the biofilm from the dish. Indeed, it was impossible for us to take the biofilm off, even by cutting the edges of the petri dish. This meant that we couldn’t coat aerogels with those biofilms. However, we saw that the GFP biofilm (fig 9.B) made some crystals and cubic structures so that the intended biofilm was less homogeneous than the silk one (fig 9.A). Overall, we managed to generate a biofilm with our recombinant silk fusion protein (mSA-silk-CBD), but not with the GFP fusion protein (mSA-GFP-CBD).
Hydrophobicity tests
To complete
References
| None |
