Part:BBa_K863101
Cellulose binding Domain of Cellulomonas Fimi Exoglucanse (Freiburg-Standard)
Cellulose binding domain of the [http://www.ncbi.nlm.nih.gov/nucleotide/327179207?report=genbank&log$=nucltop&blast_rank=3&RID=152ZCN0E01N Cellulomonas fimi ATCC 484 exoglucanase gene] in Standard 25 (Freiburg).
Usage and Biology
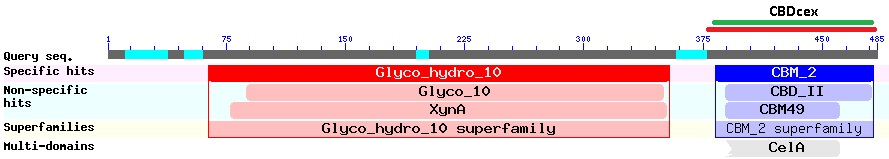
The CBDcex is an C-terminal domain at the end of the protein-sequence (Figure 2). The NCBI-BLAST identified 100 amino acids (300 bases; green bar) as the protein domain. It belongs to the Cellulose Binding Module family 2 (pfam00553/cl02709; Figure 1) and is part of of the Cellulomonas fimi ATCC 484 exoglucanase gene. In our project the domain was used N-terminal, which made the conserved linking sequence to the glycosyl hydrolase unattractive as a Linker for our fusion-proteins. For the coding sequence 12 bases (4 amino acids) more than the BLAST predicted were taken as conserved sequence upstream and 9 bases (3AS) downstream of the domain to secure natural folding of the domain.
The expressed protein would have 114 amino acids with a molecular weight of 11.4 kDa and a theoretic pI of 7.85. If the protein concentration is measured by optical density at 280 nm the extinction coefficients would be 27625 M-1 cm-1, assuming all pairs of Cys residues form cystines and 27500 M-1 cm-1, assuming all Cys residues are reduced. The [http://web.expasy.org/cgi-bin/protparam/protparam ExPASy Prot-Parameter-tool] predicted the estimated half-life 30 hours in mammalian reticulocytes, (in vitro) more than 20 hours in yeast (in vivo) and more than 10 hours in Escherichia coli (in vivo). It classified the protein as stable.
Contribution (Waterloo iGEM 2020)
Summary: CBM2a is a cellulose-binding domain that irreversibly binds to phosphoric acid-swollen cellulose (PASC) and bacterial microcrystalline cellulose (BMCC). While the protein has already been added to the iGEM parts registry with adequate description of its function, we wanted to delve deeper into its structure, functions, and possible methods of purification.
Documentation: CBM2a is a cellulose-binding domain that irreversibly binds to PASC and BMCC, but is still mobile on crystalline surfaces using three tryptophan residues on the binding face. These tryptophan residues on the binding face of the protein (W17, W54, and W72) are key to binding (McLean et al., 2000). CMB2a’s affinity was quantified by McLean et al. (2000). The team measured the change in Gibbs free energy that occurred from the binding of CBM2a with different types of cellulose. From these measurements, it seems that acid-swollen cellulose is best for maximizing binding capacity, while bacterial microcrystalline cellulose is best for maximizing affinity.
CBM2a can be used in fusion proteins, where CBM2a binds to cellulose while the other protein binds to another molecule or ion of choice. For this reason, it is an ideal candidate to be purified in a cellulose column (Zhao et al., 2015).
Structure: The main structure of the protein is a beta-barrel backbone. As mentioned previously, the aromatic amino acid residues on the flat face are involved with cellulose binding. The protein is 110 amino acids long.
Improvement (Waterloo iGEM 2020)
To facilitate the use of our desired metal-binding proteins within a column, we sought to immobilize our proteins on cellulose. We first located the cellulose binding module 2a (CBM2a) in the iGEM parts registry under BBa_K863101. While the part should be functional without modification, we decided to insert a linker between our functional metal-binding protein and the CBM. We thought it may improve the efficiency of our separations by increasing the rate at which the column could equilibrate as the binding site would be less likely to be hidden against the cellulose surface. In our literature review, a paper described a naturally occurring flexible linker between a CBM2 domain and the catalytic domain in a lytic polysaccharide monooxygenase found in Streptomyces coelicolor A3 (Courtade et al., 2018). By including this linker, part BBa_K863101 would be functionally improved for applications where the CBM is used to immobilise the fused protein on cellulose. The effect of the presence of the linker could be tested by measuring the binding constants of both the metal-binding domain and the cellulose-binding domain as described on our Measurement page. To facilitate this part for use in constructing fusion proteins, it has been formatted using BioBrick assembly standard 25.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 4
Illegal AgeI site found at 337 - 1000COMPATIBLE WITH RFC[1000]
//cds
//proteindomain/binding
| family | Cellulose Binding Modul family 2 (pfam00553/cl02709) |
| function | cellulose binding |
| origin | Cellulomonas fimi (strain ATCC 484 / DSM 20113 / JCM 1341 / NBRC 15513 / NCIMB 8980 / NCTC 7547) |
| strain | strain ATCC 484 / DSM 20113 / JCM 1341 / NBRC 15513 / NCIMB 8980 / NCTC 7547 |
| swisspro | HQ445939 |
| type | Cellulose binding domain |
| uniprot | F2XFS7 |