Part:BBa_K875009
LL 37 - Cathelicidin
The LL 37 cathelicidin is a human antimicrobial peptide.
The exact mechanism by which AMPs kill microorganisms is still under debate.
The eukaryotic toxin LL 37 was obtained by synthesis with the canonical prefix and suffix RFC10 compatible and it was optimized for E. coli expression.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Tec-Chihuahua 2019: Effect of LL 37 in E. coli growth
Team Tec-Chihuahua worked on the characterization of BBa_K875009. We realized that the few characterizations of LL 37 were all qualitative. This is why our team undertook the task of characterizing this part in order to report a quantitative absorbance study.
LL 37 has antimicrobial activity against both Gram-positive and Gram-negative bacteria. Escherichia coli is susceptible to the effects of this peptide, and since it's one of the most common chassis for recombinant protein production, the effects that LL 37 may have on the growth of E. coli when used for its expression were evaluated.
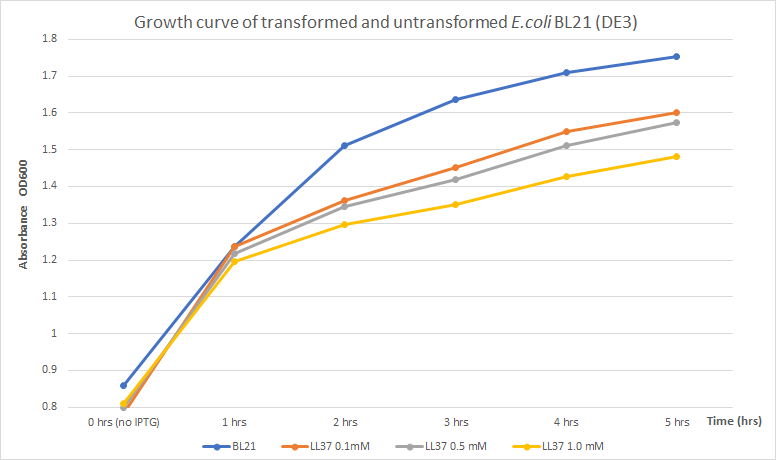
Expression of toxic proteins may adversely affect the viability of the chassis. In order to test E. coli as a viable host for LL 37 production, E. coli BL21 (DE3) cells were transformed with part BBa_K2959000 which is a composite capable of expressing LL 37 under the regulation of the T7 promoter. Three flasks with 30 mL of LB broth were inoculated with 3 mL of an overnight culture of transformed cells. Another flask with 30 mL of LB broth was inoculated with 3 mL of untransformed cells to use as a control. The four flasks were incubated at 37°C and 225 rpm until OD600 reached 0.8. The flasks with transformed cells were treated with different concentrations of IPTG (0.1, 0.5, and 1 mM) to induce protein production. Then, optical density was measured every hour for five hours to measure growth. Results were recorded in the following table and chart.
| 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | |
|---|---|---|---|---|---|---|
| Untransformed BL21 (DE3) | 0.859 | 1.238 | 1.511 | 1.636 | 1.709 | 1.752 |
| Transformed BL21 (DE3), 0.1 mM IPTG | 0.784 | 1.238 | 1.362 | 1.451 | 1.549 | 1.6 |
| Transformed BL21 (DE3), 0.5 mM IPTG | 0.80 | 1.218 | 1.346 | 1.418 | 1.51 | 1.573 |
| Transformed BL21 (DE3), 1.0 mM IPTG | 0.81 | 1.197 | 1.297 | 1.351 | 1.427 | 1.481 |
The results obtained show a distinct difference in absorbance, which correlates to culture growth, between untransformed and transformed E. coli. After 5 hours of incubation, untransformed BL21 (DE3) (blue line) indicated an OD600 of 1.752. Previous data, compared to transformed BL21 (DE3) with LL37 (yellow line) with the highest induction condition and time (1mM of IPTG and 5 hours), shows an OD600 of 1.481 which denotes a reduction in growth of approximately 15.5% . Untransformed cells grew better indicating that LL 37 production has a negative effect on bacterial growth. Concentration of IPTG and absorbance showed an inversely proportional relationship. Higher concentrations of IPTG must induce a larger production of LL 37 and, due to its toxicity towards its host, it may have caused this partial inhibition. Thus, expression of LL 37 reduces bacterial growth; however, no significant reduction in total growth was perceived. In conclusion, inhibition is not significant enough to consider expression of LL 37 in E. coli BL21 (DE3) non-viable.
//collections/antimicrobial
//collections/probiotics
//collections/probiotics/biocontainment
| None |
