pMC_0_7+8_panS_specResLVL1
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal XbaI site found at 1199
Illegal PstI site found at 3734 - 12INCOMPATIBLE WITH RFC[12]Illegal PstI site found at 3734
Illegal NotI site found at 1
Illegal NotI site found at 5738 - 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 4897
Illegal XhoI site found at 1249 - 23INCOMPATIBLE WITH RFC[23]Illegal XbaI site found at 1199
Illegal PstI site found at 3734 - 25INCOMPATIBLE WITH RFC[25]Illegal XbaI site found at 1199
Illegal PstI site found at 3734
Illegal NgoMIV site found at 48
Illegal NgoMIV site found at 190 - 1000COMPATIBLE WITH RFC[1000]
B A S I C P A R T S
Cyanobacterial shuttle vectors
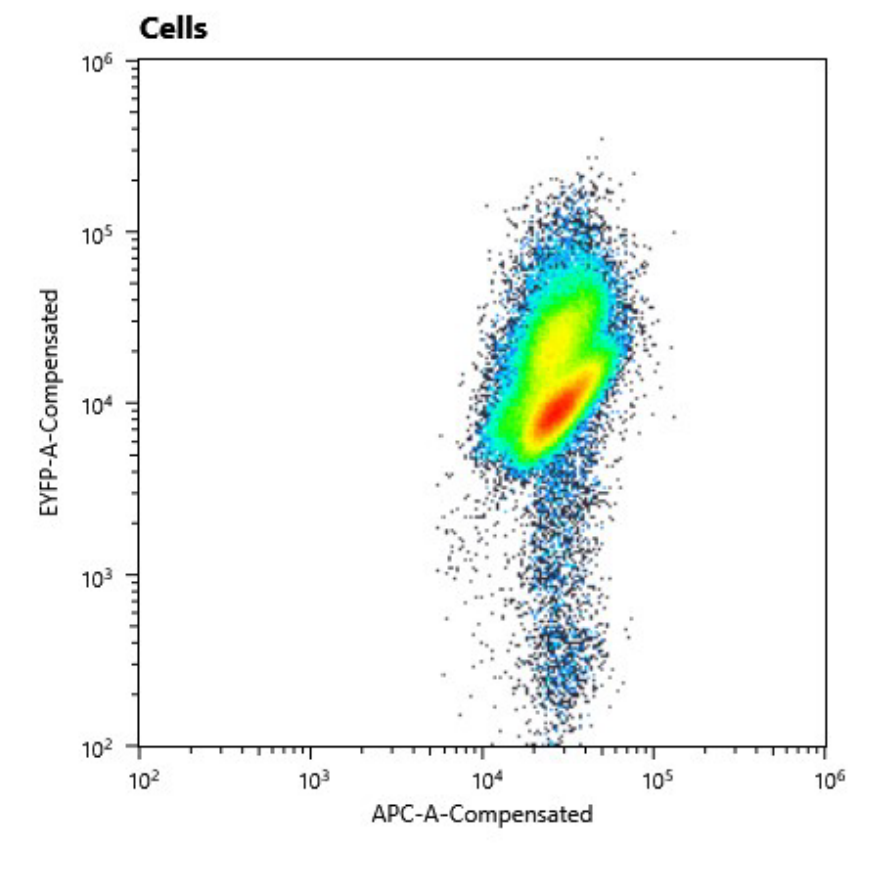
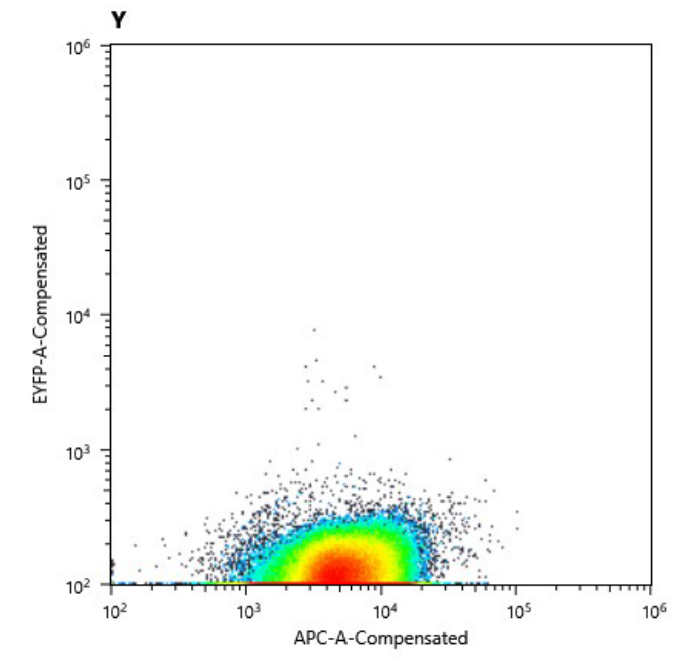
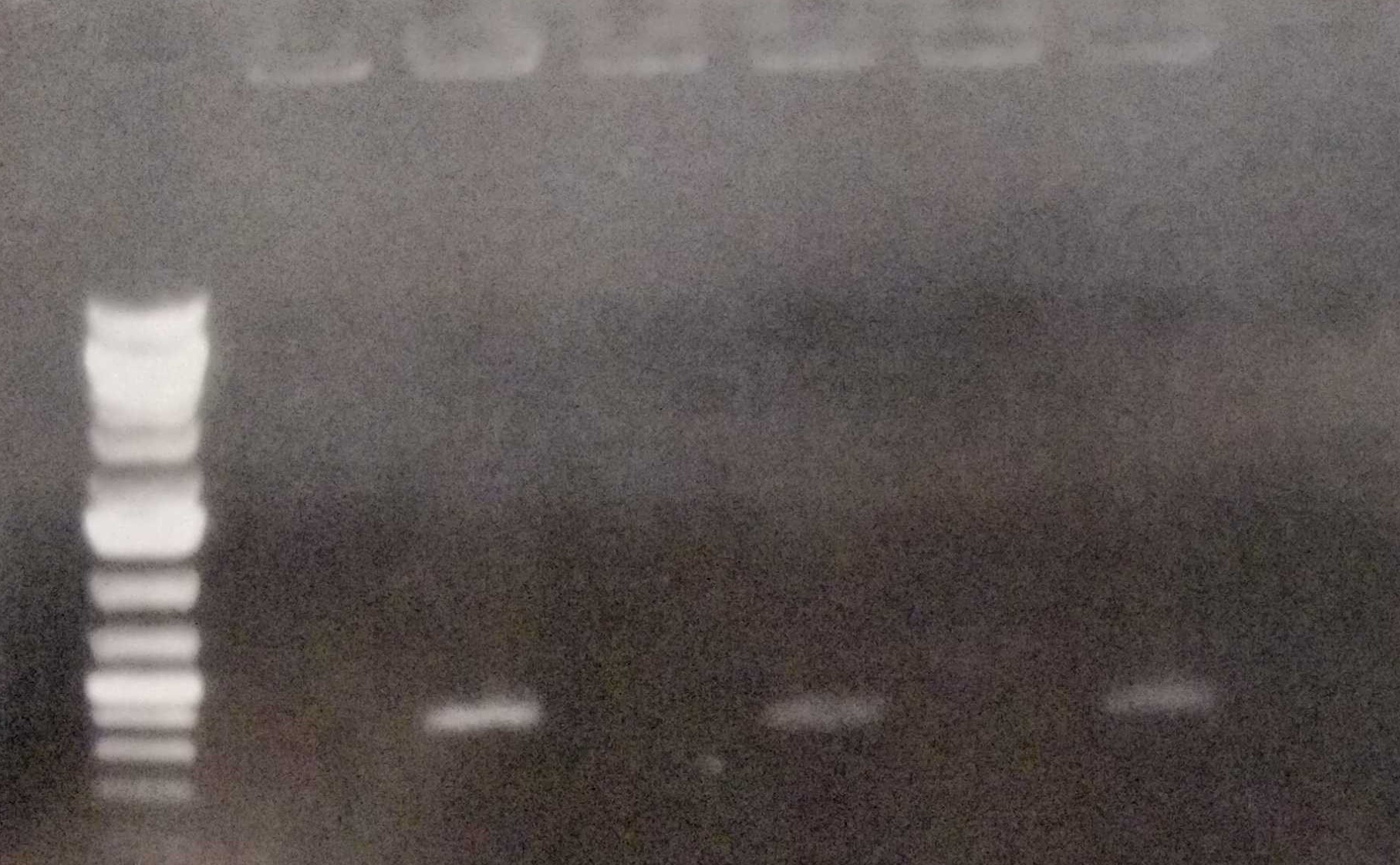
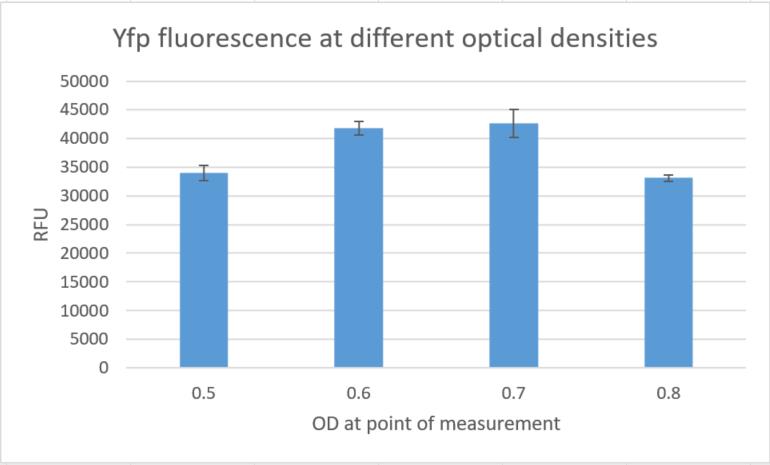
As we have already clarified in the description part, self replicating shuttle vectors are essential for many workflows, as the gene expression levels are higher and non of the tedious selection processes that come with genomic integrations have to be done.On our road to the modular vector we were seeking, we firstly cured our own S. elongatus UTEX 2973 strain of its pANS plasmid. This was done by transforming the pAM4787 vector, which holds a spectinomycin resistance as well as a YFP cassette (Chen et al., 2016). Due to plasmid incompatibility - explained here in our design section [Link to shuttle vector design] - and because antibiotic pressure is applied, the pANS plasmid was over time cured from the strain, which then just kept the pAM4787 plasmid. Transformation was done by conjugation with the pRK2013 plasmid in DH5ɑ and the pAM4787 in HB101. Both were grown to an OD600≈0.5, washed in LB and mixed with S. elongatus which was grown to late exponential phase and then washed in BG11. We could clearly show, that the conjugant strain bears the pAM4787 plasmid if selective pressure is held up.


This part is contained in the Green Expansion, a range of parts from iGEM Marburg 2019that enables users of the Marburg Collection 2.0 to design MoClo compatible vectors for cyanobacteria as well as to engineer the genome of several cyanobacterial species.
The Green Expansion
The Green Expansion is an addition of parts to the Marburg Collection 2.0 (See: Design of the Marburg Collection) that features the world's first MoClo compatible shuttle vector for cyanobacteria. BBa_K3228069
The Green Expansion also offers all the parts needed for the genomic integration of one or multiple genes in cyanobacteria. This M.E.G.A. (Modularized Engineering of Genome Areas) kit convinces with a striking flexibility and a very intuitive workflow for the de novo assembly of your plasmid of choice. It encompasses five different neutral integration sites to choose from: three conventional sites frequently used in the cyanobacterial community (NSI to NSIII) as well as our own rationally designed artificial Neutral integration Site options a.N.S.o. 1 and 2 (See: Finding new artificial Neutral integration Site options).These sites show no transcriptional activity from neighboring regions according to RNA-seq data and are therefore completely orthogonal. Additionally we offer four different antibiotic markers to use (chloramphenicol, gentamicin, spectinomycin and kanamycin). With the Green Expansion up to 20 genes can be introduced into a cyanobacterial strain.
Thanks to the flexible design this expansion can also be used for the genomic modification of any chassis after the introduction of a new species specific LVL 0 integration sites to our Marburg Collection 2.0. As the workflow to build new homologies is a bit more intricate compared to the one pot on step assembly of our other parts due to the internal BsmBI cutting site, we described the workflow for that in our design section (See: Design of neutral integration sites).
The Green Expansion proves a valuable addition to our Marburg Collection 2.0 and to the iGEM Registry of Standard Biological Parts. It services users of our chassis and other cyanobacterial strains with a useful tool for genomic modifications but it also contributes a shell that can be used to modify any other model organism as well.
Compability
These parts are compatible with the RCF [1000] standard and can be used in any part collection that uses the PhytoBrick standard of overhangs. For more information we recommend to head over to Design of the Marburg Collection iGEM Marburg 2018.
Parts of the Green Expansion





