Part:BBa_K3017062
CRISPRi sgRNA for rfp DNA binding - transcription template with terminator
This part is a transcription template CRISPRi sgRNA for rfp DNA binding with a terminator (BBa_B1002). In our project, the terminator terminates the in vitro transcription of single-guide RNA (BBa_K3017001 and BBa_K3017002) without forming secondary structures with the RNA itself, avoiding functional interferences. Users can add their own choice of promoter before this part for in vitro transcription.
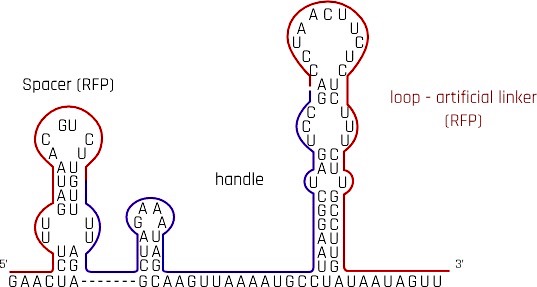
dCas9 protein is directed to the specific DNA locus by a single-guide RNA (sgRNA), where it binds to suppress downstream gene expression. With reference to the research[1] on reversible CRISPRi switch, we redesigned the traditional sgRNA by adding an artificial linker behind crRNA and tracrRNA and modified the 3-component-sgRNA to suit our suppression purpose. Our design of sgRNA is compatible with spCas9.
Spacer - crRNA
crisprRNA(crRNA) is also commonly referred to as the spacer. When choosing the target binding region, we considered mainly 2 factors, namely the location of the PAM sequence and the suppression effect upon binding.
The research[1] shows CRISPRi suppression effect is the strongest 35nt upstream start codon of the coding region. However, the area upstream of our coding region is a generic constitutive promoter. To avoid non-specific binding, we compromised the suppression efficiency and chose a region shortly after the start codon, where suppression is only a few percent weaker than the ideal region. To accommodate the PAM sequence in BBa_E1010 mrfp (CGG, 11nt into the gene), the spacer is arranged on the opposite DNA strand, 14nt into the gene. When sgRNA with spacer complementary to mrfp sequence is transcripted, it binds with dCas9 to repress the expression of mrfp.
Handle - tracrRNA
tracrRNA is an RNA loop that acts as a handle for dCas9 to hold onto. So that the dCas9 protein is delivered to the target site together with the sgRNA. Experiments have proved that tracrRNA is strictly required for Cas9-mediated DNA interference both in vitro and in vivo[4]. The tracrRNA forms a loop on the sgRNA after transcription to provide a scaffolding site for the dCas9 to form a duplex with the spacer.
Loop - artificial linker
Destroying the secondary structure of the handle in sgRNA could theoretically cause dissociation of the dCas9 protein from the sgRNA, together, removing the suppression effect. The study mentioned above had proved this hypothesis correct. The team[1] then tried to design an artificial linker, which also forms a loop as a secondary structure, after the handle. After several trials and modifications, the research team discovered that extending the artificial loop, i.e. destroying the secondary structure, could further increase the derepression.
A corresponding antisense RNA (asRNA), K3017004, is responsible for reversing the repression effect on rfp induced by this sgRNA. Part of the antisense is complementary with the artificial linker of this sgRNA.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |