Part:BBa_K2812004
Coding sequence for trunctated Lysostaphin fused to His-tagged HlyA
The biobrick contains the coding domain for truncated lysostaphin, based on the coding sequence derived from BBa_K748002. This was fused to the C-terminal sequence of Hemolysin A (HlyA) via a thrombin linker to allow secretion of the lysostaphin. TU-Eindhoven 2018 used this part to secrete lysostaphin from Escherichia coli to kill Staphylococcus aureus biofilms for the treatment of wound infections. For more information about our project, please visit our [http://2018.igem.org/Team:TU-Eindhoven wiki].
Usage & Biology
Lysostaphin
Lysostaphin is an antimicrobial agent produced by Staphylococcus simulans. targets the cell wall peptidoglycan found in certain Staphylococci by cleaving its cross-linking pentaglycine bridges. Among others, it is effective for degrading Staphylocuccus aureus biofilms.1 The encoding part of the lysostaphin has been derived from BBa_K748002, made by iGEM Harbin 2012 and is also used by iGEM Stockholm 2016. iGEM Eindhoven 2018 codon optimized this lysostaphin construct. Lysostaphin belongs to the major class of antimicrobial proteins and peptides known as bacteriocins. Bacteriocins are proteins or peptides produced by bacteria, displaying a bactericidal activity against other subpopulations of bacteria.3 The cell wall degradation capability of lysostaphin derives from its endopeptidase activity on pentaglycine cross-bridges in the peptidoglycan layer. Specific cleavage between the third and fourth glycine residue leads to the destruction of the peptidoglycan layer and subsequent lysis of the bacteria.
HlyA
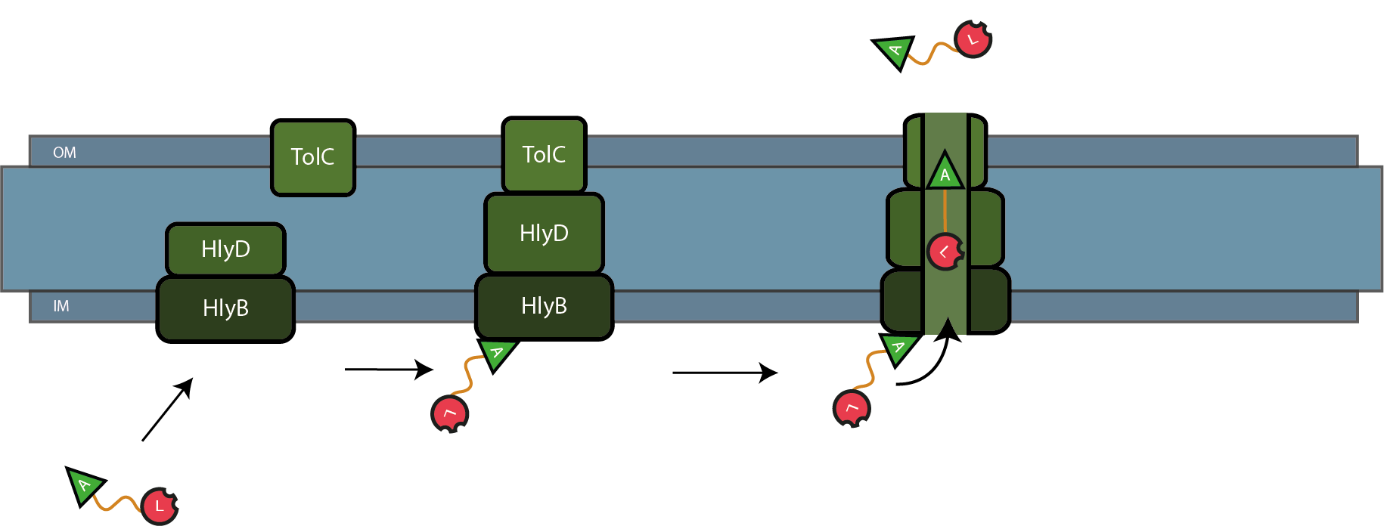
The C-terminal sequence of Hemolysin A (residues 807-1024 of the E. coli HlyA gene) functions as a non-cleavable signal peptide for protein translocation via the Type I secretion pathway of Gram-negative bacteria. Here, HlyA is fused to the lysostaphin for its secretion from E. coli BL21 (DE3) cells. . In Type I secretion, single step transport of the target protein occurs from the cytoplasm to the extracellular environment. The HlyA is fused to the C-terminus of the target protein.2 It is a 23-kDa signal sequence that targets proteins for secretion via Type I secretion pathway. HlyA is secreted into the medium in a TolC and HlyB/D-dependent manner.4 It is recognized by the membrane translocation complex composed of HlyB and HlyD, which together with the TolC protein, will form a pore through the membrane.5 This will lead to the secretion of HlyA-containing fusion proteins. Figure 1 illustrates the steps involved in the type I secretion of lysostaphin as an HlyA fusion protein.
Thrombin linker & His-tag
The lysostaphin and HlyA are linked via a thrombin linker. It is a short peptide sequence which can be cleaved by the enzyme thrombin, resulting in the removal of the HlyA domain. This avoids interference with the functionality of lysostaphin. C-terminal to the HlyA domain, a His-tag (6x repeated amino acid histidine) is attached. The His-tag can be used for detection of the fusion protein by e.g. Western Blot, but also for protein purification purposes as it facilitates binding to a nickel affinity column.
Fusion protein
The combination of all components described above, lead to a construct that can be (continuously) secreted by E. coli if the HlyB/D coding sequences have been co-transformed. Running the medium containing the fusion protein over a nickel affinity column and subsequent thrombin linker cleavage allows for the easy isolation of purified truncated lysostaphin.
Experimental Characterisation by TU Eindhoven (2018)
Cloning
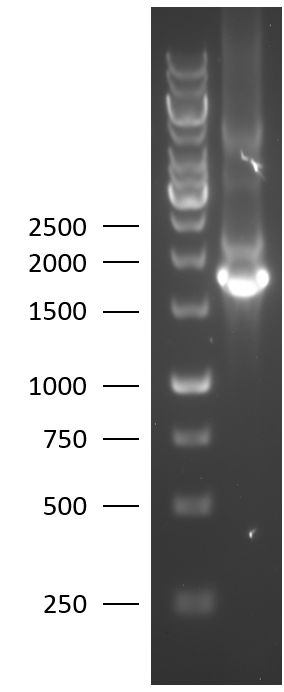
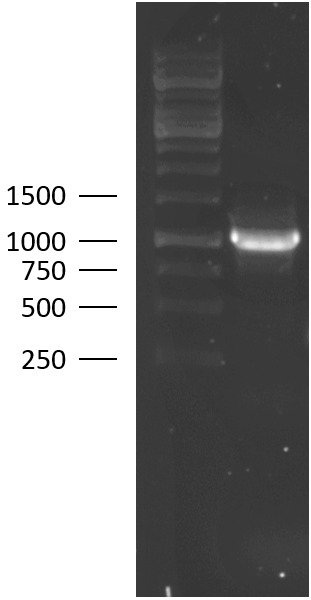
TU Eindhoven 2018 has characterized the biobrick (BBa_K2812004) at both the DNA and the protein level. First, the lysostaphin-thrombin linker-HlyA-His-tag construct was synthesized by IDT and subsequently double digested and assembled into the digested linearized pSB1C3 backbone via ligation. The ligated construct was successfully transformed into E. coli NovaBlue, followed by a colony PCR using the VF2 and VR primers to investigate if the correct length has been inserted in the vector. The mixture was ran on a 1% agarose gel as can be seen on the right. The observed length of the brightest band corresponds with the expected length of X basepairs, confirming that the desired construct has been succesfully ligated in pSB1C3 and subsequently transformed in E. coli NovaBlue. Next, the colonies with the correct insert were cultured in LB before a plasmid purification by a miniprep. The isolated plasmid DNA was sent for Sanger sequencing and the sequence could be confirmed.
Protein Expression
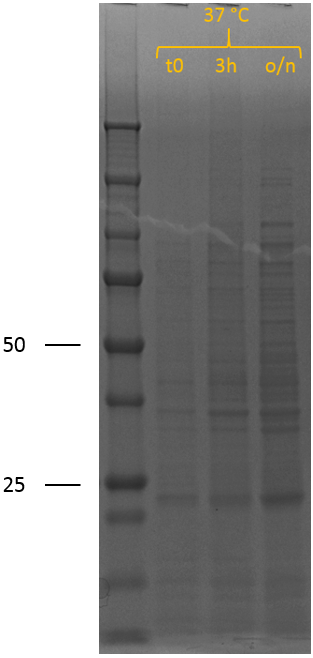
After the successful characterisation of the biobrick at the DNA level, protein expression experiments were performed. To allow expression of the construct by the addition of IPTG, it was assembled behind the T7 promotor of iGEM Bielefeld 2011 BBa_K525998 using restriction digestion by EcoRI and PstI followed by ligation. We have also included this construct as a new composite part in the registry, see BBa_K2812005 for a more detailed characterisation at the DNA level. The biobrick was successfully transformed into BL21 (DE3), after which a culture was set up at 37 ┬░C. A sample was taken before induction (t0). The cultures were induced at an OD600 of 0.5-0.8 by adding 0.5 mM IPTG to induce expression of the recombinant protein, also at 37 ┬░C. Samples were taken 3 hours (3h) after induction and after overnight incubation (o/n). SDS samples were prepared and loaded onto a polyacrylamide gel to yield the SDS-PAGE results that can be seen on the right.
In the uninduced sample (t0), no bands indicative of overexpression of (any) protein can be observed. 3 hours after induction of protein expression, two new bands can be seen; one at 50 kDa and one at 25 kDa. After overnight, the band at 50 kDa has disappeared completely and the band around 25 kDa has increased in intensity. The biobrick is expected to show up around 50 kDa and explains that band, confirming induction of recombinant protein expression. However, the band around 25 kDa and the disappearance of the band at 50 kDa is not expected, but can be explained by lysostaphin accumulating in the cytosol. The thrombin linker used contains two times a GGGGS repeat. The substrate sequence of lysostaphin is GGGGG. However, it is known that the bacterial species S. simulans incorporates serine residues at the third and fifth position in the cell wall cross bridges (GGSGS), resulting in a 1000-fold decrease in susceptibility to lysostaphin as lysostaphin cannot hydrolyse glycylserine and serylglycine bonds.1 Lysostaphin is thus still able to cleave these linkers, although at a lower rate. If the thrombin linker between lysostaphin and HlyA is cleaved by another lysostaphin enzyme, it yields two proteins of 27 kDa (lysostaphin) and 23 kDa (HlyA). The band around 25 kDa and the disappearance of the 50 kDa band can thus be explained by lysostaphin cleaving the thrombin linker.
Functionality Experiments
Cell lysates & Medium
To prove that this cleavage does not occur rapidly intracellularly preventing secretion of the lysostaphin domain (as HlyA is lost), functional experiments were performed in collaboration with the PAMM foundation, which is the regional center of infectious diseases and pathology in South-East Brabant in the Netherlands.
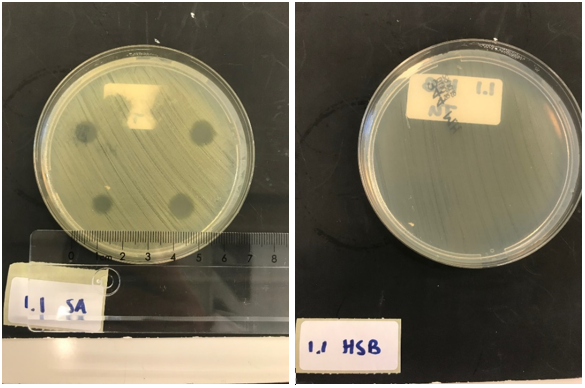
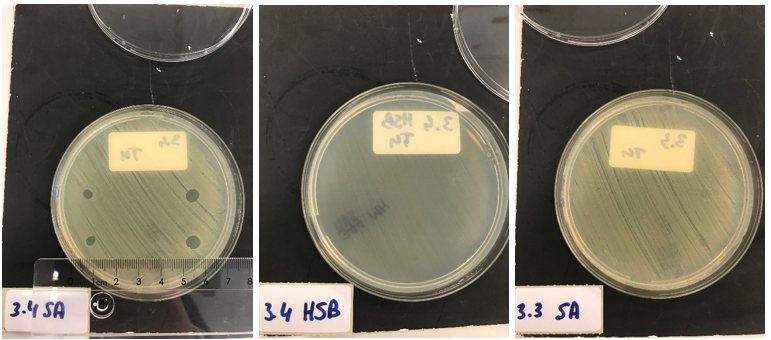
First, to test the production and effectiveness of lysostaphin, BL21 (DE3) cells were transformed with the plasmid containing the T7 promotor from iGEM Bielefeld 2011 (BBa_k525998) and our biobrick, and induced overnight. Only the production of lysostaphin is expected here, since the HlyB/D proteins required for type I secretion are missing. The cell lysate was taken from the culture and washed thoroughly, to remove any present antibiotic to exclude antibiotics as the source of bactericidal activity against S. aureus. S. aureus and Streptococcus agalactiae were plated on separate Mueller-Hinton agar plates and 3 (left top and bottom) and 5 (right top and bottom) ┬ÁL of E. coli cell lysate was applied. As a control, the same experiment was performed but now with a biofilm of Streptococcus agalactiae (HSB). This is another strain of gram positive bacteria that is not susceptible to lysostaphin but will be affected if e.g. traces of antibiotic are still present.
After an overnight incubation with the cell lysate of our lysostaphin producing E. coli, the following results were obtained (3 ┬ÁL top and bottom left; 5 ┬ÁL top and bottom right):
Clear halo formation on the S. aureus plate can be observed, indicating the production and activity of lysostaphin in the cell lysate. No halo formation was observed on the Streptococcus agalactiae plate, ÔÇÖconfirming that the results observed are caused by the bactericidal activity of lysostaphin produced by our E. coli, and not by possible remains of antibiotics.
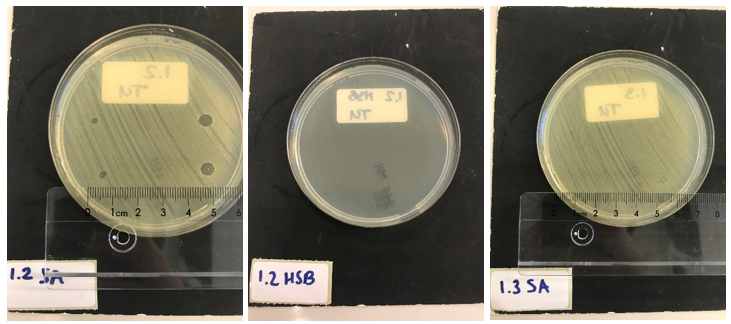
Next, we tested the functionality of the secretion system. Since the pSB1C3 biobrick plasmid and the pSTV plasmid containting the HlyB/D secretion proteins had overlapping antibiotic resistances, the biobrick was assembled into a pBAD vector under control of an AraC promotor inducible by ATC. E. coli BL21 (DE3) cells were transformed with both the HlyB/D plasmid and our newly assembled biobrick plasmid. Both plasmids were then simultaneously induced. After four hours, the proteins were isolated from the medium and washed extensively to remove any residues of antibiotics. A part of the medium was concentrated 10X and a part was concentrated 30X. Similarly, the pellets were lysed and washed. As a control, BL21 (DE3) cells expressing both a modified construct without lysostaphin but only HlyA and the HlyB/D construct were tested. These bacteria can only secrete HlyA. After induction, samples from these bacteria were prepared according to the same protocol. S. aureus and Streptococcus agalactiae were plated on separate Mueller-Hinton agar plates to which 3 and 5 ┬ÁL of E. coli cell lysate was applied. After an overnight incubation with the cell lysate of our lysostaphin producing E. coli, the following results were obtained (3 ┬ÁL top and bottom left; 5 ┬ÁL top and bottom right):
30x concentrated medium (3 ┬ÁL top and bottom left; 5 ┬ÁL top and bottom right):
Clear halo formation in 1.2 SA confirms that the lysostaphin in the cell lysate is active. Absence of halo in 1.2 HSB indicates that the bactericidal activity against S. aureus is not due to antibiotics remnants being present but has to be due to the Lysostaphin-HlyA construct. Absence of halo formation in 1.3 SA confirms that the bactericidal activity against S. aureus is due to lysostaphin, and not the HlyA domain. The same conclusion as the construct under control of the T7 promotor. 3.4 SA shows that the concentrated medium of the induced Lysostaphin-HlyA has bactericidal acitivity against S. aureus, while the HlyA construct 3.3 SA has no bactericidal acitivty. The medium of Lysostaphin-HlyA has no bactericidal acitivty against Streptococcus agalactiae (3.4 HSB). This confirms that lysostaphin is successfully secreted in the medium by our engineered E. coli bacteria and that the bactericidal activity observed against S. aureus is due to the lysostaphin and not HlyA or other components in the medium.
Co-culture of lysostaphin-secreting E. coli and Staphylococcus aureus or Streptococcus agalactiae
Finally, as an ultimate experiment for confirming the secretion of the lysostaphin construct by E. coli, a co-culture of S. aureus or Streptococcus agalactiae with E.coli BL21 (DE3) on Mueller-Hinton agar plates was performed. The plates were covered with the inducers to induce expression of the biobrick or HlyA and the HlyB/D secretion proteins. First S. aureus or Streptococcus agalactiae were plated on the inducer-agar. Subsequently, drops of uninduced BL21 (DE3) cultures were put on top of this film. This resulted in the following observations:
In 2.1 SA, clear halo formation around the BL21 (DE3) colonies can be observed, indicating secretion of lysostaphin having a bactericidal effect on S. aureus. 2.1 HSB clearly shows that on another Gram positive bacterial strain, lysostaphin does not show any antimicrobial activity. As a final control, BL21 (DE3) only expressing HlyA and HlyB/D (without lysostaphin), was co-cultured with S. aureus (see 2.2 SA). Around these E.coli colonies, no halo formation can be observed again illustrating that the lysostaphin component of the fusion proteins contains the bactericidal effect selectively against S. aureus, and HlyA is not toxic.
Functional improvement of BBa_K748002
To demonstrate the functional improvement over the original truncated lysostaphin BBa_K748002 created by HIT-Harbin 2012, we intended to assemble our biobrick (BBa_K2812004) with the T7 promotor BBa_K525998, allowing protein expression by induction with IPTG. Since the part BBa_K2144001 from iGEM Stockholm 2016 is this exact assembly, we requested their part from the iGEM registry and we transformed it successfully in E. coli BL21 (DE3). Colony PCR was used to pick colonies carrying the correct insert length. After subsequent culturing and miniprep, we sent the plasmid DNA for sanger sequencing and could confirm the sequence.
Transformed E. coli BL21 (DE3) bacteria were cultured overnight at 37 ┬░C, followed by protein induction at an OD600 of 0.5-0.8 by adding 0.5 mM IPTG, also at 37 ┬░C. Before induction, a cell pellet sample was taken as control. Cell pellet samples were collected after 3 hours and after overnight induction. All samples were prepared for SDS-PAGE. The SDS gel can be seen on the right. No clear overexpression of a band around 27 kDa, the expected length of truncated lysostaphin, can be seen. As no convincing expression of the confirmed construct can be observed, our codon optimisation improved expression of the lysostaphin domain considerably. However, in our eyes the most valuable functional improvement of this part over BBa_K748002 is the ability for lysostaphin to be secreted, which is obviously not possible for the BBa_K748002 part lacking the HlyA domain.
Overall conclusion
We have demonstrated that the designed Lysostaphin-HlyA fusion protein gets successfully secreted by E. coli via Type I secretion if HlyB/D is co-expressed in the bacteria. The Lysostaphin-HlyA biobrick has a bactericidal effect against S. aureus and the construct is not toxic for other, non-susceptible bacteria like Streptococcus agalactiae. Importantly, the construct is not active against E. coli and secretion enables continuous release. Additionally, the use of cell lysis to release lysostaphin is no longer required, functionally improving the truncated lysostaphin construct from iGEM Harbin 2012 which required cell lysis for release in their system. Besides release, isolation of lysostaphin with this newly designed construct is also easier as purification can be performed directly from the medium, avoiding the requirement of cell lysis and decreasing purification problems. Unexpectedly, self-cleavage of the thrombin linker is observed if lysostaphin is allowed to concentrate due to cleavage at the GGGGS sites of the linker. To minimize self-cleavage, partial inhibition of lysostaphin can be achieved by adding excess Zinc to the culture medium.1 Alternatively, the design of the linker could be changed to avoid repeats of glycine residues.
Sources
1) Tossavainen, H., Raulinaitis, V., Kauppinen, L., Pentik├Ąinen, U., Maaheimo, H., & Permi, P. (2018). Structural and Functional Insights Into LysostaphinÔÇôSubstrate Interaction. Front Mol Biosci.
2) Thomas, S., Holland, I. B., & Schmitt, L. (2014). The Type 1 secretion pathway ÔÇö The hemolysin system and beyond. Molecular Cell Research, 1629-1641.
3) Bastos, M. d., Coutinho, B. G., & Coelho, M. L. (2010). Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals (Basel) , 1139ÔÇô1161.
4) Gray, L., Baker, K., Kenny, B., Mackman, N., Haigh, R., & Holland, I. (1989). A novel C-terminal signal sequence targets Escherichia coli haemolysin directly to the medium. J Cell Sci Suppl., 45-57.
5) Wandersman, C., & Delepelaire, P. (1990 ). TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. , 4776-4780.
6) Kawabata, E., Kikuchi, K., Urano, Y., Kojima, H., Odani┬ž, A., & Nagano, T. (2005). Design and Synthesis of Zinc-Selective Chelators for Extracellular Applications. J. Am. Chem. Soc., 818ÔÇô819.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 1351
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 126
- 1000COMPATIBLE WITH RFC[1000]
//cds
//cds/enzyme
//cds/enzyme/lysis
//cds/membrane
//cds/membrane/lysis
//chassis/prokaryote/ecoli
//collections/biofilm
//plasmid/expression
//plasmid/expression/t7
//plasmidbackbone/expression/inducible
//plasmidbackbone/proteinfusion
//plasmidbackbone/synthesis
//proteindomain
//proteindomain/cleavage
//proteindomain/degradation
//proteindomain/linker
//proteindomain/localization
| None |