Part:BBa_K1893010
Constitutive STAR (J23119+STAR)
This is the STAR construct under control of the constitutive promoter j23119. When transcribed, it produces the STAR-antisense RNA (pAD1.A5), which can bind to the sense STAR-target attenuator sequence (pAD1.S5). Thus, only in the presence of STAR-antisense, transcription and expression of coding sequences downstream to the STAR-target is activated.
Usage and Biology
For designing a small transcriptional activating RNA through an anti-terminator mechanism, Chappell et. al. investigated transcriptional terminators from different plasmids and of different lengths and sequences. Of these, the sense pAD1 plasmid attenuator sequence no. 5 (pAD1.S5) and its associated STAR-antisense sequence no. 5 (pAD1.A5) pair gave the highest (94-fold) activation of the reporter gene, SFGFP [Chappell et al., 2015]. Thus, we used the pAD1.S5 sequence as our STAR target-encoding DNA and the pAD1.A5 sequence as the STAR-antisense-encoding DNA.
STAR binds a sense target RNA sequence, the pAD1 plasmid attenuator sequence, which is fused upstream of the gene of interest. In the absence of STAR, the pAD1 plasmid attenuator sequence forms an intrinsic hairpin structure, preventing RNA polymerase from transcribing the gene and thus acting as a terminator of transcription. However, when STAR is produced, it binds to the target sequence and interferes with the hairpin formation, allowing transcription of the downstream gene.
Characterisation data
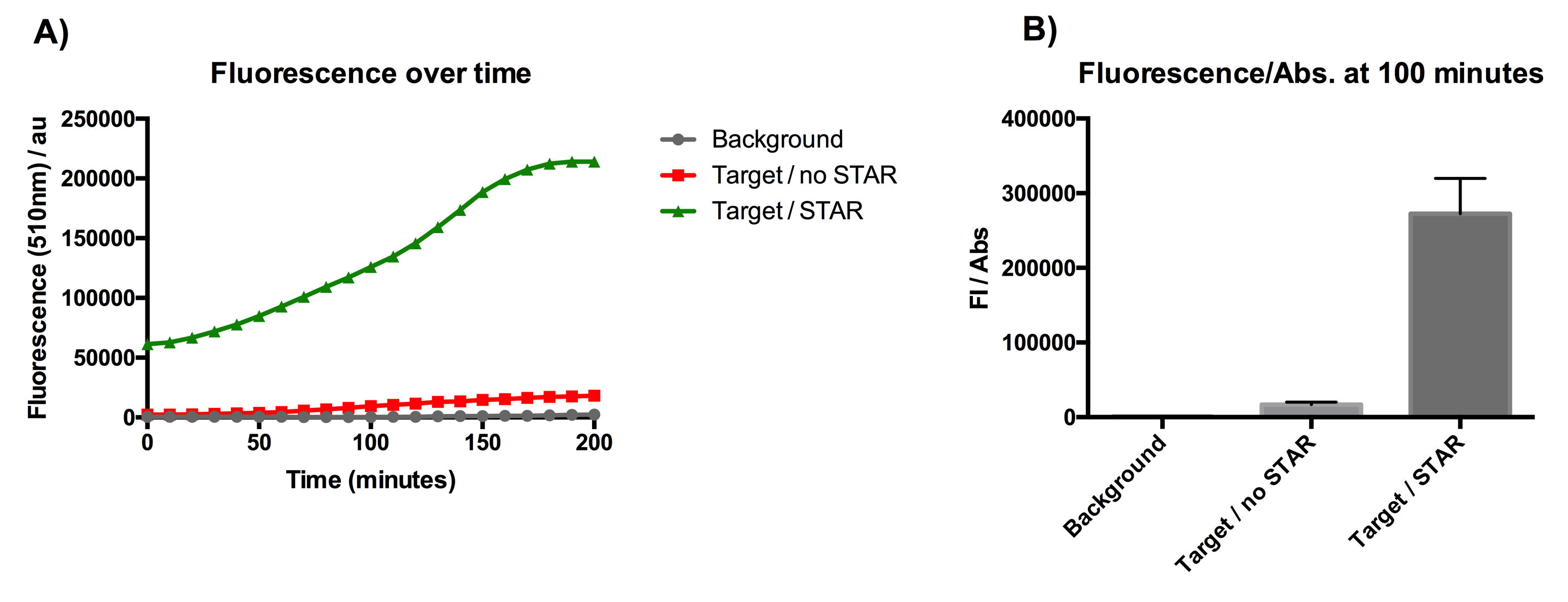
Figure 1: Characterisation of STAR system in TOP10 E. coli cells. (A) Normalised fluorescence monitored over time for cell lines incorporating the STAR system in the absence or presence of transcribed STAR molecules (B) Normalised endpoint fluorescence (100 minutes) for cell lines in the absence or presence of STAR molecules. We used the two-plasmid system described in the Experimental Design for characterisation experiments involving STAR. For the absence of STAR condition, the plasmid did not include STAR sequence but just the J23119 promoter. Normalised fluorescence was calculated by dividing fluorescent signal by the O.D.600 value of the culture. Background was determined by the use of DH10B cells with no plasmid transformed. Error bars represent standard deviation from 3 technical repeats.
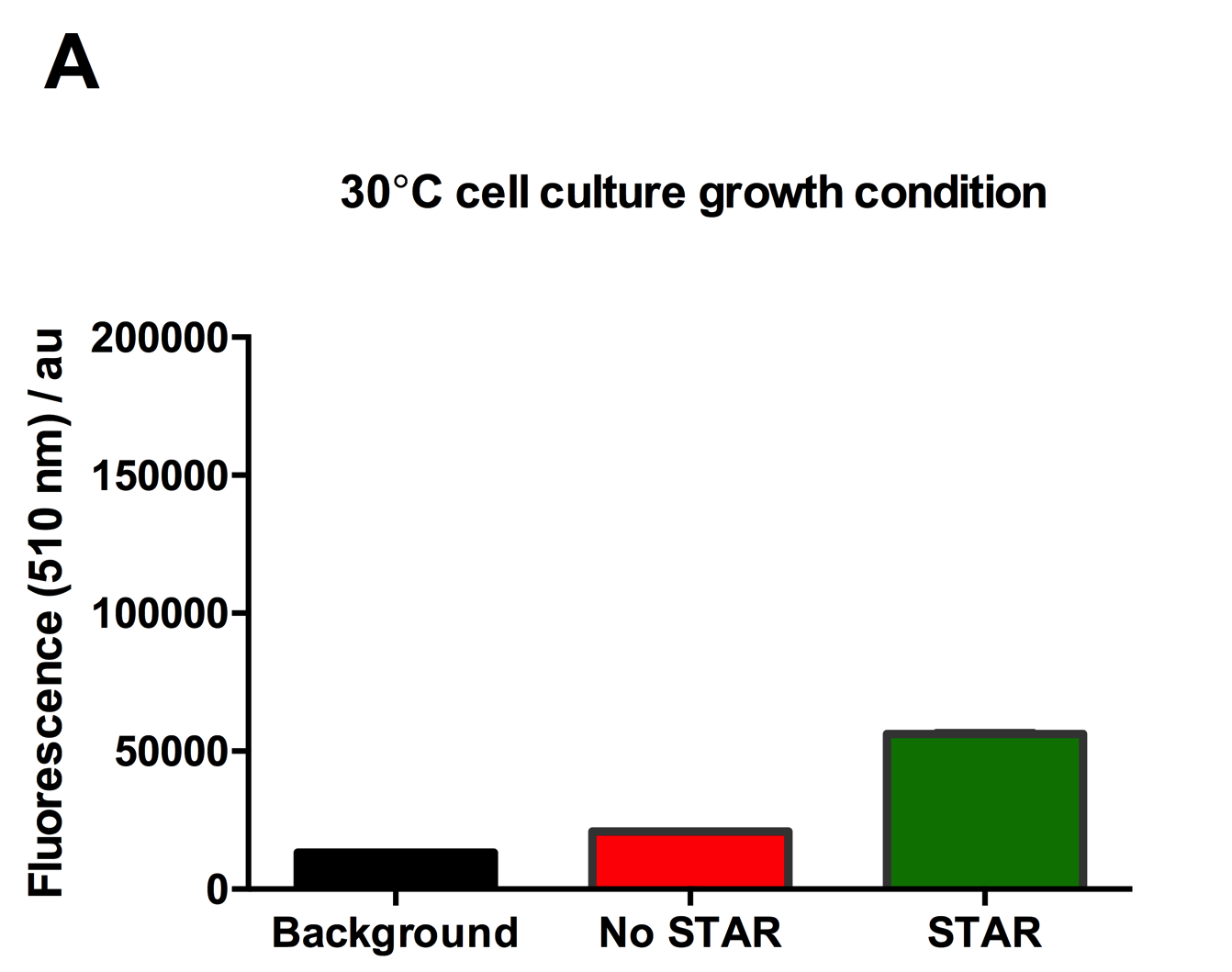
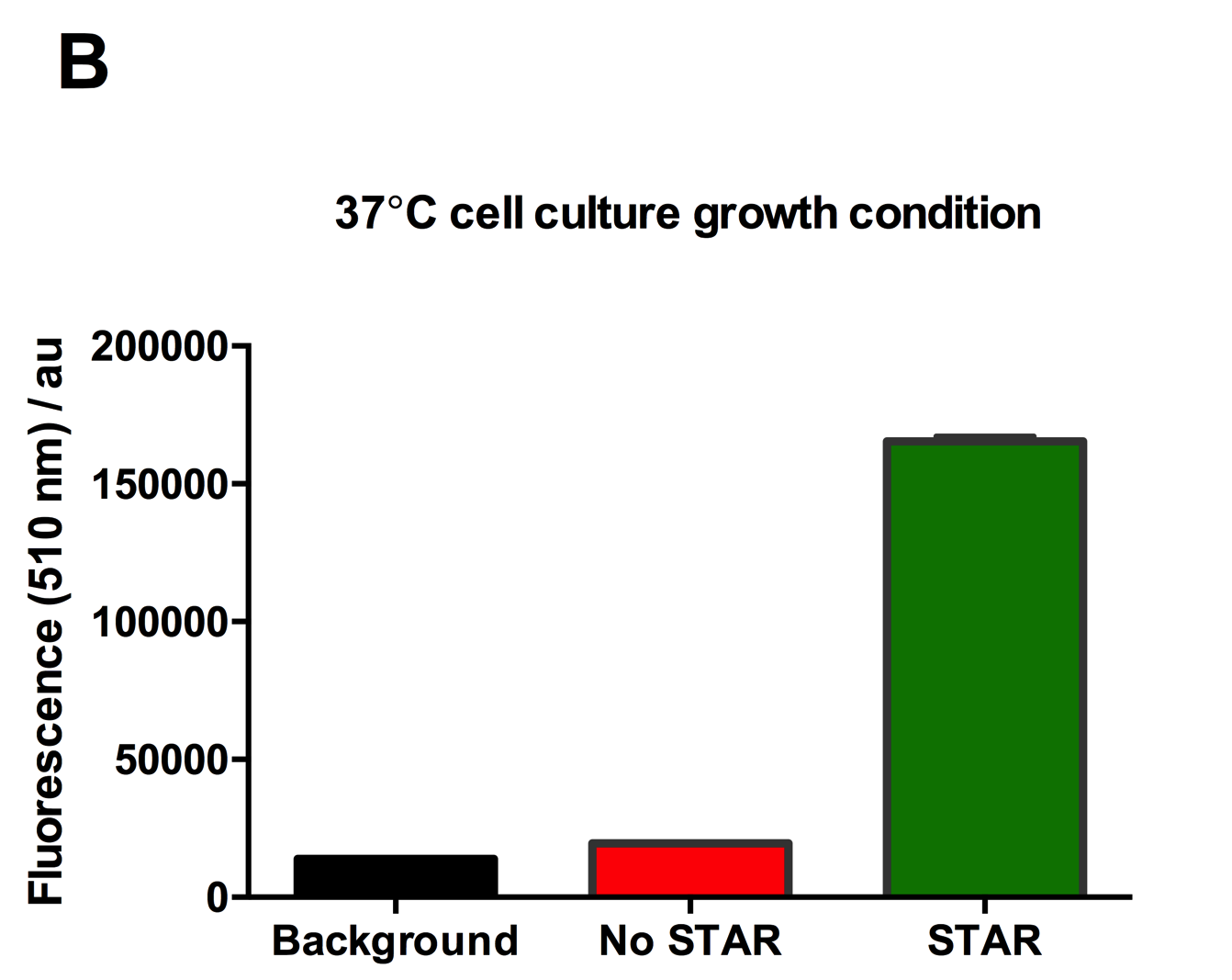
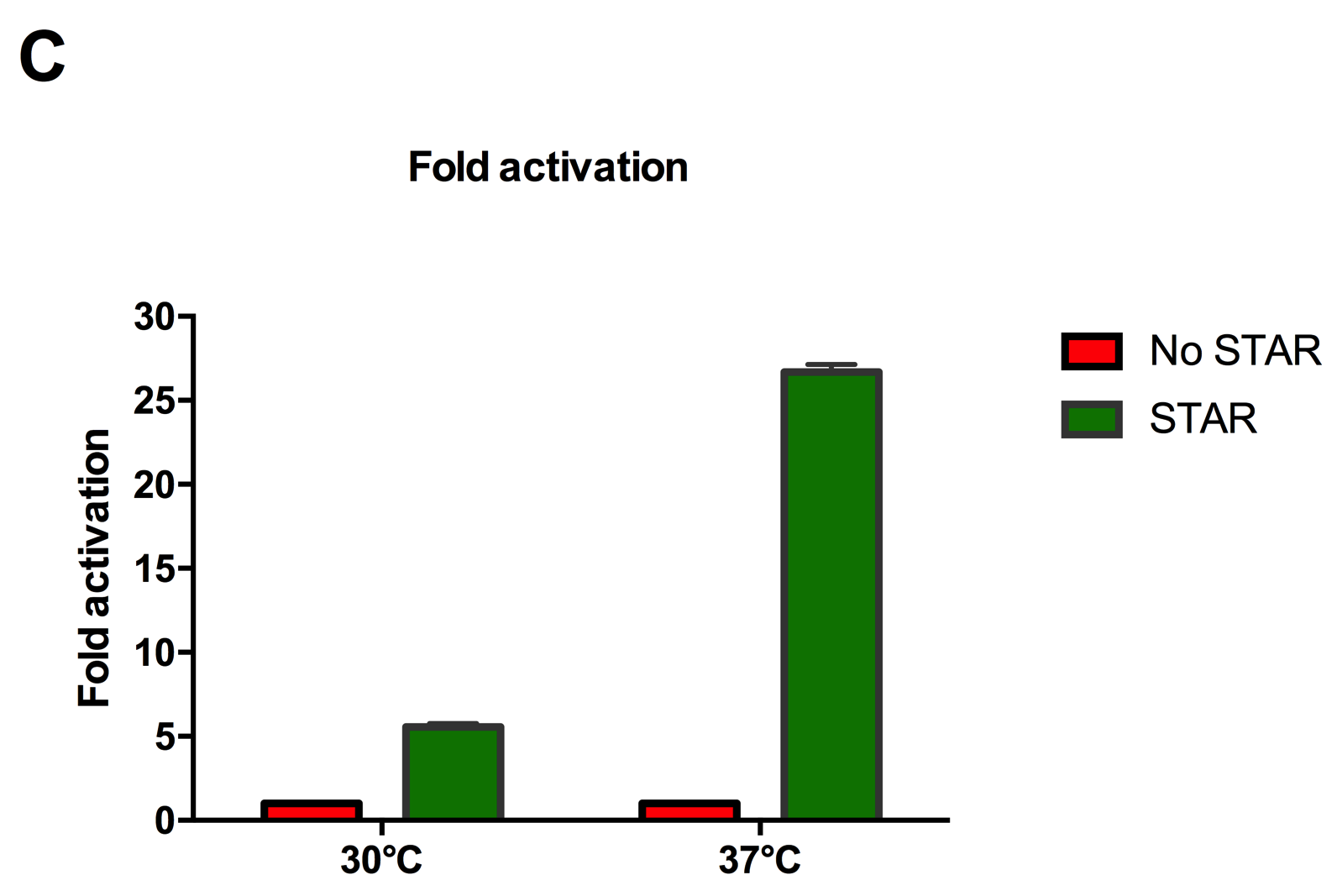
Figure 2: Characterisation of STAR system in TOP10 E. coli cells at different temperatures. (A) Cell culture fluorescence at 30°C (B) Cell culture fluorescence assay at 37°C. (C) Fold activation SFGFP expression in presence of STAR. We used the two-plasmid system described in the Experimental Design for characterisation experiments involving STAR. For the absence of STAR condition, the plasmid did not include STAR sequence but just the J23119 promoter. The autofluorescence background control used is E. coli Top 10 cells with no reporter plasmid. Error bars represent standard deviation from 3 technical repeats.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
| None |