Part:BBa_K1592003
Mcfp3 with LIP2 prepro
Mcfp-3 is foot protein secreted from Mytilus californianus. The protein is of significance to the formation of byssus to help mussels permanently or temporarily tether to the surface of solid surface of reef or ship-body. This coherent substance shows the excellent adhesion performance with no substitute under water for sustaining the repeating wash of waves and having no toxicity. The signal tag, LIP2 prepro(BBa_K1592000), fused to Mcfp3 can lead the co-translational translocation of heterologous protein Mcfp-3 to be secreted out of the cell to achieve its function, during the progress of which the peptide will be deleted in the Golgi apparatus to eliminate the interference to the secreting protein. And hexahis-tag added between LIP2 prepro and Mcfp3 can therefore be used for protein purification.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 100
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Characterization
LIP2 prepro, fused to Mcfp3, can lead the co-translational translocation of heterologous protein Mcfp-3 to be secreted out of the cell to achieve its function. To verify the function of Mcfp3, we use the promoter hp4d to express it.
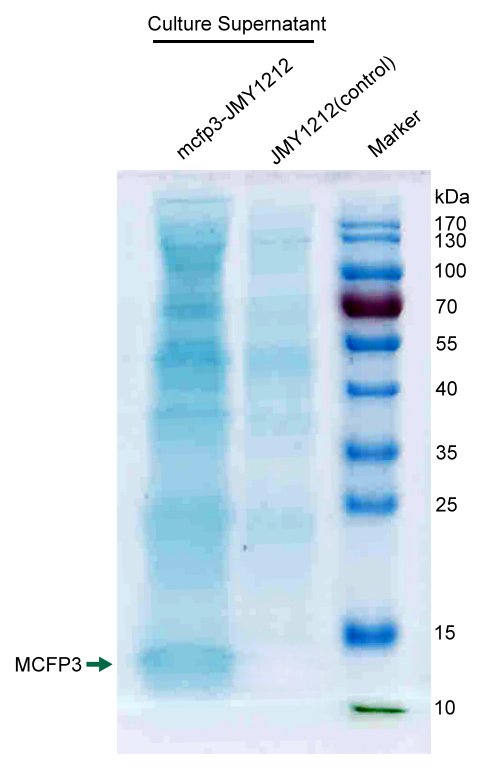
SDS-PAGE
We concentrated cell culture solution of test Y.lipolytca JMY1212 cells transformed LIP2 prepro-Mcfp3 plasmid and control wildtype cells, and then separated proteins by SDS-PAGE.
Figure shows an obvious ~12kDa protein bands of LIP2 prepro-Mcfp3 in test lane, which cannot be found in control lane. This result proves that LIP2 prepro signal peptide can successfully lead the flocculating proteins Mcfp3 into surrounding environment.
To verify mcfp3’s function, we did flocculating test on slides. The concentrated cell culture medium of Mcfp3 transformed cells, BSA and PBS buffer were added on one slide respectively for overnight. After that, the slide was washed by ddH2O, stain coomassie brilliant and rinse.
The figure shows that compared to the control BSA that was washed off, Mcfp3 protein remained on slide obviously, which indicate this protein can flocculate and adhere on rough surface automatically.
Test of sands cementation with cells transformed Si-tag1+2+3 and Mcfp3

To test the cementation ability of our genetic engineering yeast, we conducted a laboratory test of sands cementation. As we can see in Figure A, 40 gram quartz sands mixed with genetic engineering yeast or control wildtype cells were loaded into each glass column, while solution carrying oxygen, calcium and culture nutrient was supplied in tubes under the impulse from peristaltic pump.
After 24 hours treatment, we dehydrated our sands columns in drying oven, and then took out the sands from the column. We can see the sands treated with control wildtype Yarrowia lipolytica JMY1212 are still scattered, only a few small solids can be found, these may be induced by the respiratory action of cells. However, by the treatment of genetic engineering yeast (Si-tag+Mcfp3), the sands aggregated obviously, and we can even obtain an intact sand cylinder (Fig B). We further compared the treated sands under microscope, we can find that the quartz sand granules treated with genetic engineering yeast aggregated together (Fig C) while the quartz sand granules treated with wildtype cells are still dispersed.
This results shows that our genetic engineering yeast actually works well to make the silica particles form certain intact structure, which fits our cementation function hypothesis and design. We can also find in the figure that there are some small holes in the sand cylinder. This special structure indicated the balance between CO2 released from cell respiration and calcium sedimentation caused by released CO2. this is the final and vital step of the genetic engineering cell cementation process. In some cementation utilization, this structure is very important. For example, in desert sands solidation treatment, the multiporous structure will eliminate the potential compaction risk that may reject plants to grow. In artificial reef construction of aquafarm, the multiporous structure will also offer harbors to all kinds of marine lifes.
References:
ZHAOH, ROBERTSONNB, JEWHURSTSA, et al. Probing the adhesive footprints of Mytilus Californianus byssus [J]. JBiol (16):11090-11096. Chem, 2006, 281
| None |