Part:BBa_K1159001:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1159001
iGEM TU Eindhoven 2015
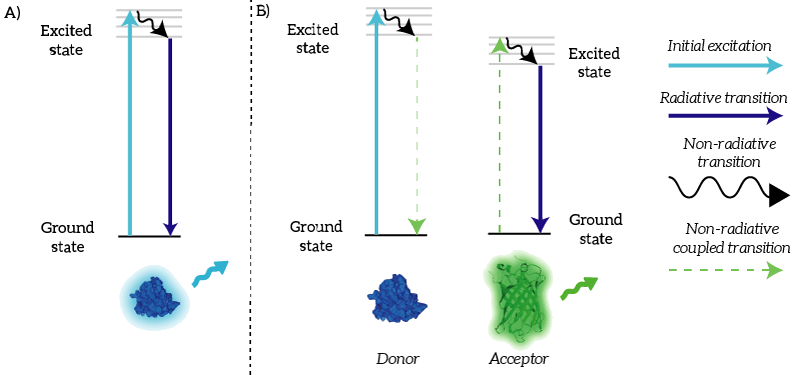
We as iGEM TU Eindhoven 2015 used NanoLuc in a BRET sensor together with mNeonGreen. Bioluminescence Resonance Energy Transfer is a physical process which can take place between fluorophores when they are in close proximity (1-10nm) [1]. An electron which has been transferred to its ‘excited state’ falls back to its ‘ground state’. The energy that is released by the electron falling back to its ground state is normally released in the form of light. In the case of Resonance Energy Transfer, however, the energy of the electron falling back to its ground state is coupled to a transition of an electron in the RET Acceptor from its ground state to the excited state (see Figure 1).
Figure 1: A) Normally, an excited electron falls back to its ground state under the emission of light (a radiative transition). B) In the case of BRET, an excited electron in the donor falls back to its ground state. This transition is coupled to the excitation of an electron in the acceptor. This excited electron falls back normally under the emission of light.
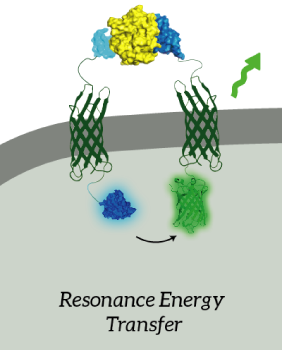
The main goal of our project was to design a universal, modular biosensor that could detect molecules by using aptamers. NanoLuc worked very well for us. We've inserted NanoLuc in a pETDuet-1 vector together with Outer Membrane Protein X (OmpX) and a BsoBI-linker, which is a long and flexible GGSGGS-linker with two BsoBI restriction sites. Several experiments gave very strong results for NanoLuc. Together with an OmpX-mNeonGreen construct, BRET can take place between the two fluorophores.
Figure 2: The OmpX-NanoLuc construct together with the OmpX-mNeonGreen construct. When in close proximity, BRET takes places from NanoLuc to mNeonGreen.
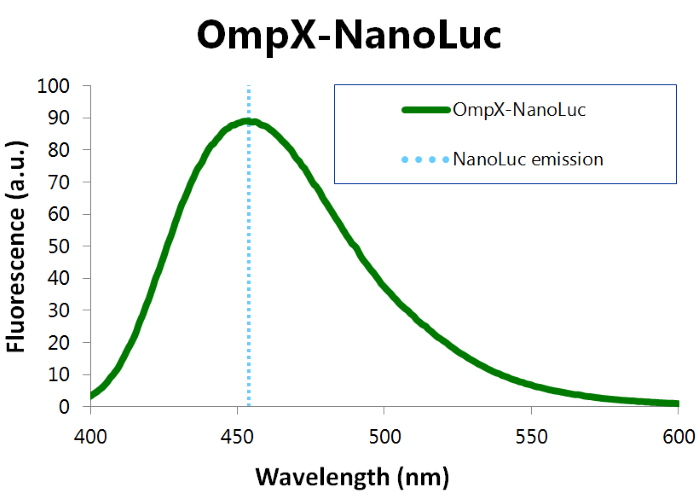
We've characterized NanoLuc by performing a bioluminescence assay with the OmpX-NanoLuc construct. This construct was inserted in the pET-Duet-1 vector, which has a T7-promoter. IPTG induces this T7-promotor. The bioluminescence assay gave the following results (see Figure 3).
Figure 3: Bioluminescence results of our OmpX-NanoLuc construct.
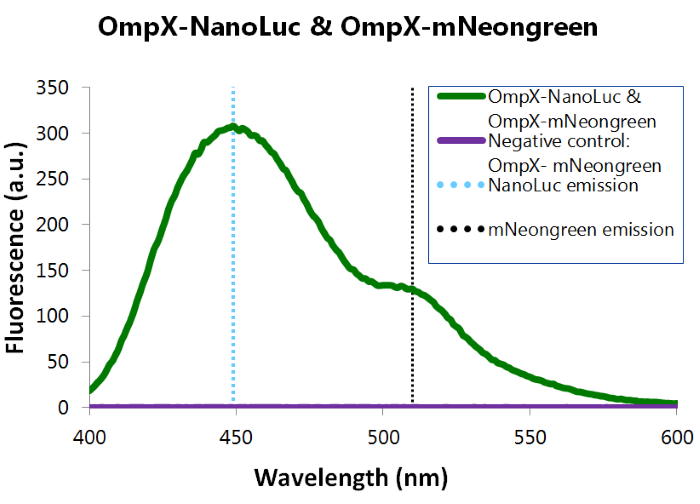
We've also performed a bioluminescence assay for the construct that both contains OmpX-NanoLuc and OmpX-mNeonGreen. This gave the following results (see Figure 4). From this it can be concluded that both the construct were present, that NanoLuc worked well as a BRET donor and that mNeonGreen worked well as a BRET acceptor.
Figure 4: Bioluminescence results of our complete construct, containing both NanoLuc and mNeonGreen.
References:
Medintz I. and Hildebrandt N., Eds., FRET - Förster Resonance Energy Transfer. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2013.
User Reviews
UNIQb6719158e285db0c-partinfo-00000000-QINU
|
•••••
iGEM TU Eindhoven 2015 |
This part works very well. We used NanoLuc in a BRET sensor, connected to OmpX with a BsoBI-linker. When furimazine is added, NanoLuc will work as a donor, which means that the energy of an electron falling back to its ground state is coupled to a transition of an electron in the RET Acceptor from its ground state to the excited state. We used mNeonGreen as acceptor in this BRET sensor. For more information and further characterization, see the Applications section above. |
UNIQb6719158e285db0c-partinfo-00000002-QINU