Part:BBa_K863022
bhal laccase from Bacillus halodurans with constitutive promoter J23100, RBS and HIS tag
bhal laccase with constitutive promoter J23100, RBS and HIS tag
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal XbaI site found at 38
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 8
Illegal NheI site found at 31 - 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 219
- 23INCOMPATIBLE WITH RFC[23]Illegal XbaI site found at 38
- 25INCOMPATIBLE WITH RFC[25]Illegal XbaI site found at 38
- 1000COMPATIBLE WITH RFC[1000]
First some trials of shaking flask cultivations were made with various parameters to identify the best conditions for production of the His tagged laccase Lbh1 from [http://www.dsmz.de/catalogues/details/culture/DSM-18197.html?tx_dsmzresources_pi5 Bacillus halodurans C-125 ] named BHAL. Due to inactivity of the enzyme in the cell lysate a purification method was established (using Ni-NTA-Histag resin). BHAL could not be detected by SDS-PAGE (theoretical molecular weight of 56 kDa) or activity test by using the BioBrick BBa_K863020 and E. coli KRX as expression system. Due to this results the new BioBrick BBa_K863022 was constructed and expressed E. coli Rossetta-Gami 2. With this expression system the laccase could be produced and analysed via SDS-PAGE. A small scale Ni-NTA-column was used to purify the laccase. The fractionated samples were tested regarding their activity with ABTS and showed ability in oxidizing ABTS. A scale up was not yet performed.
Contents
Cultivation, Purification and SDS-PAGE
Cultivation
The first trials to produce the Lbh1 - laccase from Bacillus halodurans (named BHAL) were performed in shaking flasks with various flask designs (from 100 mL-1 to 1 L flasks, with and without baffles) and under several conditions. The varied parameters in our screening experiments were temperature (27 °C,30 °C and 37 °C), concentration of chloramphenicol (20-170 µg mL-1), induction strategy (autoinduction and manual induction with 0,1 % rhamnose) and cultivation time (6 to 24 h). Furthermore we cultivated with and without 0.25 mM CuCl2 to provide a sufficient amount of copper, which is needed for the active center of the laccase. E.coli KRX was not able to produce active BHAL under the tested conditions, therefore another chassis was chosen. For further cultivations E. coli Rosetta-Gami 2 was transformed with BBa_K863012, because of its ability to translate rare codons. BHAL was produced under the following conditions:
- flask design: shaking flask without baffles
- medium: [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Materials#LB_medium LB]-Medium
- antibiotics: 60 µg mL-1 chloramphenicol and 300 µg mL-1 ampicillin
- temperature: 37 °C
- cultivation time: 24 h
Purification
The cells were harvested and resuspended in Ni-NTA-equilibration buffer, mechanically lysed by sonification and centrifuged. After preparing the cell paste the BHALlaccase could not be purified with the 15 mL column, because of the column was not available. For this reason a small scale purification (6 mL) of the supernatant of the lysate was performed with a [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Production#Syringe_method 1 mL Ni-NTA-column]. The elution was collected in 1 mL fractions.
SDS-PAGE
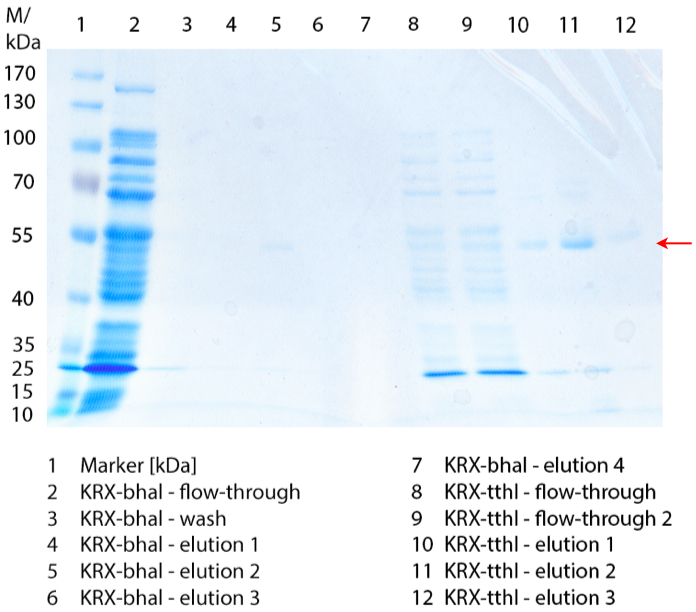
In Figure 1 the different fractions of the purified cell lysate of E. coli Rosetta-Gami 2 with BBa_K863022 are shown in a SDS-PAGE. BHAL has a molecular weight of 56 kDa. In lane 5, which corresponds to the elution fraction 2, a faint band of 56 kDa is visible. Therefore the fractions were further analysed by activity test and MALDI-TOF.
Since Regionals: 12L Fermentation of E. coli Rosetta-Gami 2 with BBa_K863022
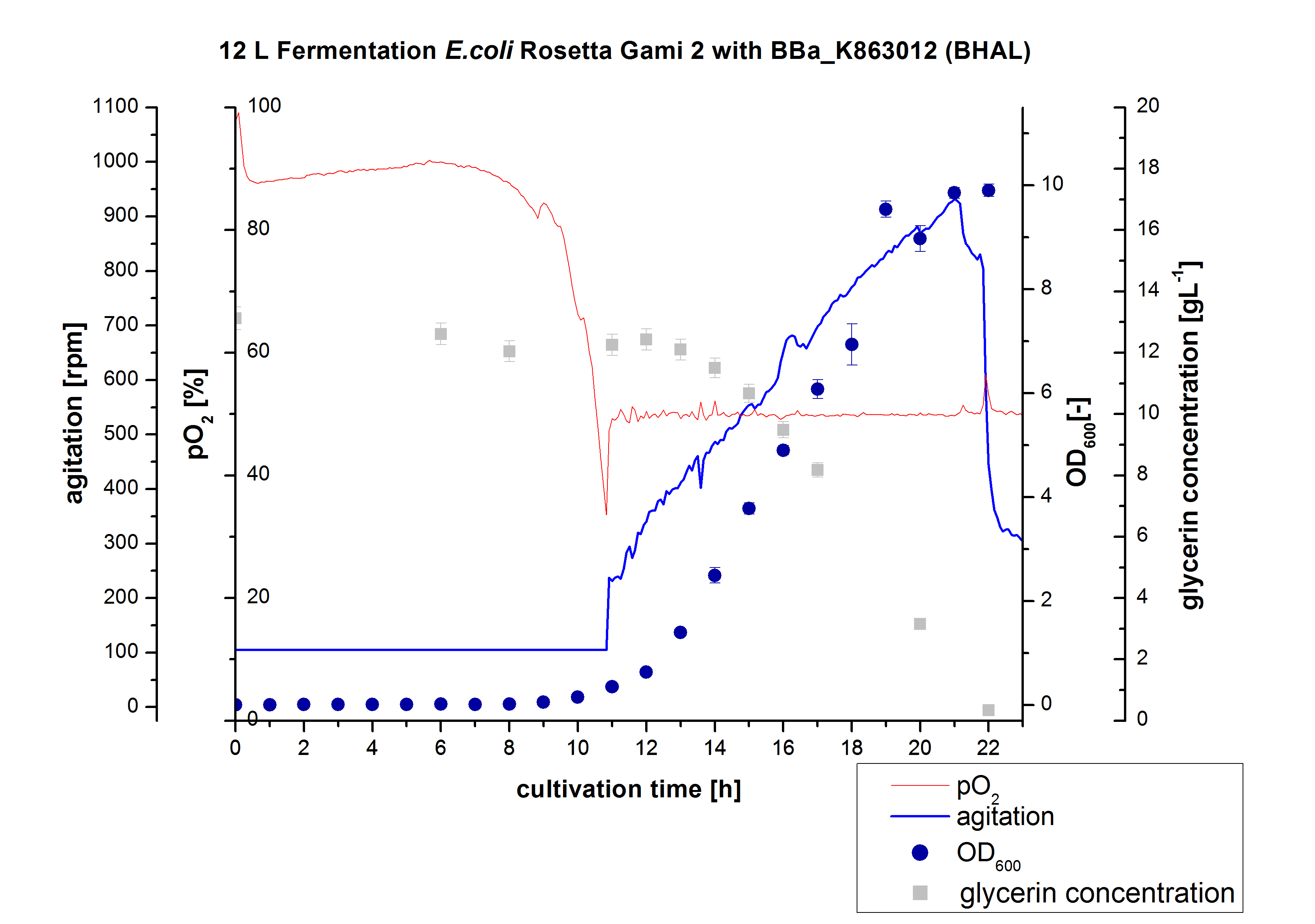
After measuring the BHAL activity a scale-up was performed and E. coli Rosetta-Gami 2 with BBa_K863022 was cultivated in a Bioengineering NFL 22 fermenter with a total volume of 12 L. Agitation speed, pO2 and OD600 were determined as well as the glycerin concentration. The data are illustrated in Figure 2. This time [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Materials#HSG_Autoinduction_medium HSG autodinduction medium] was used to produce more biomass. Due to the change of media and to a low amount of cells for inoculation there was a long lag phase of nearly 10 hours. During this phase the glycerin concentration was approximately constant. The following cell growth caused a decrease of glycerin concentration and of pO2. After 11 hours the value fell below 50 %, so that the agitation speed increased automatically. After 21 hours the deceleration phase started and therefore the agitation speed decreased. The maximal OD600 of 9.9 was reached after 22 hours, when the cells entered the stationary phase. The glycerin was completely consumed. The cells were harvested at this time. It might have been better to cultivate a few hours longer.
Since Regionals: Purification of BHAL
The harvested cells were resuspended in [http://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA equilibration buffer] and mechanically disrupted by [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Production#Mechanical_lysis_of_the_.28bio-reactor.29_cultivation homogenization]. The cell debris were removed by centrifugation and microfiltration via [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50]. The supernatant of the cell lysate was concentrated with [http://www.millipore.com/catalogue/module/C7493 Millipore Pellicon XL 50] with 10 kDa and loaded on the Ni-NTA column (15 mL Ni-NTA resin) with a flow rate of 1 mL min-1 cm-2. Then the column was washed with 10 column volumes (CV) [http://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA equilibration buffer]. The bound proteins were eluted by an increasing [http://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA elution buffer] step elution from 5 % (equates to 25 mM imidazol) with a length of 80 mL, to 50 % (equates to 250 mM imidazol) with a length of 80 mL and finally to 100 % (equates to 500 mM imidazol) with a length of 90 mL. This strategy was chosen to improve the purification caused by a step by step increasing Ni-NTA-elution buffer concentration. The elution was collected in 10 mL fractions. In figure 3 only the UV-detection signal of the wash step and the elution are shown, this is because of the high UV-detection signal of the loaded samples and to simplify the illustration of the detected product peak. A typical chromatogram of purified laccases is illustrated here. The chromatogram of the BHAL elution is shown in Figure 5:
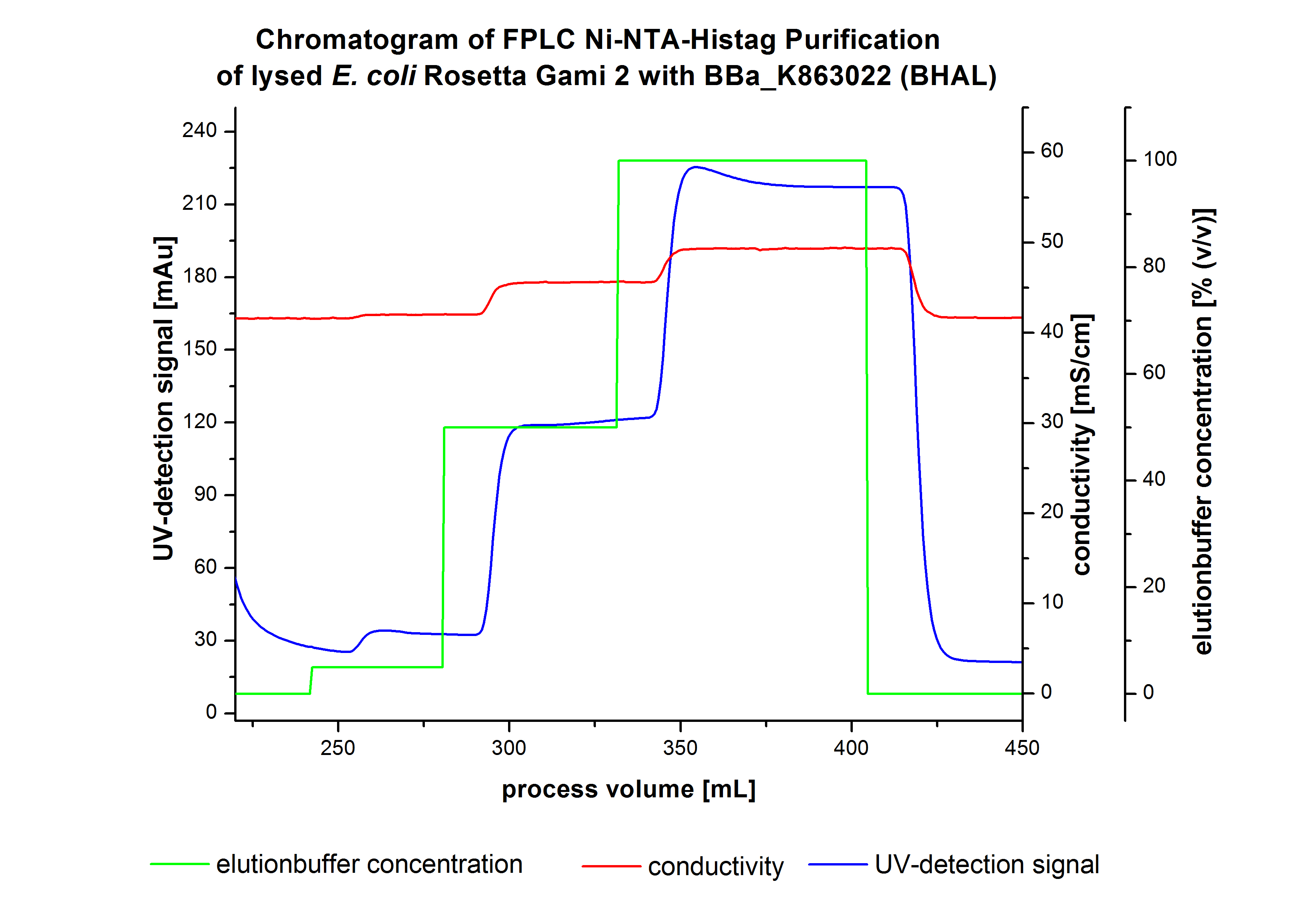
The chromatogram shows two distinguished peaks. The first peak was detected at a Ni-NTA-equilibration buffer concentration of 5 % (equates to 25 mM imidazol) and resulted from the elution of weakly bound proteins. Contrary to our expectations, the chromatogram shows the second distinguished peak. This peak was detected at a [http://2012.igem.org/wiki/index.php?title=Team:Bielefeld-Germany/Protocols/Materials#Buffers_for_His-Tag_affinity_chromatography Ni-NTA-equilibration buffer] concentration of 100 % (equates to 500 mM imidazol) and resulted from the elution of bound protein. Earlier measurements of other bacterial laccases showed that the elution of these laccases begins with a elution buffer concentration of 50 %(equates to 250 mM imidazol). One explanation of this result could be a low concentration of the produced BHAL. Consequently all elution fractions were analyzed by SDS-PAGE to detect BHAL. In the chromatogram no further peaks were detected. The following increasing UV detection signal by increasing concentration of the eltutionbuffer results from the rising imidazol concentration of the Ni-NTA elution buffer. The corresponding SDS-PAGES are shown in Figure 4.
Since Regionals: SDS-PAGE of protein purification
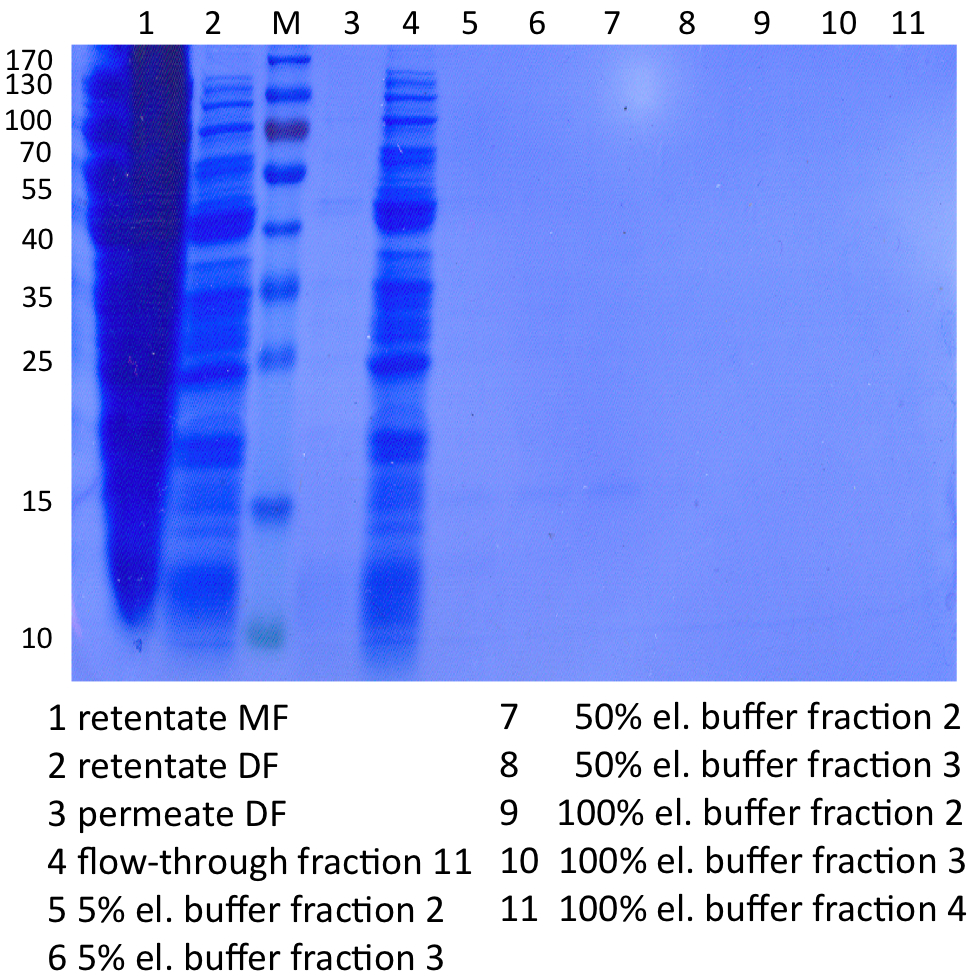
In Figure 4 the SDS-PAGE of the Ni-NTA purification of the lysed E.coli Rosetta-Gami 2 culture containing BBa_K863022 is illustrated. It shows the permeate and retentate of microfiltration and diafiltration respectively, several fractions of flow-through, wash and the elutions with different buffer concentrations respectively. The selected samples were taken where peaks were seen in the chromatogram. The His-tagged BHAL has a molecular weight of 56 kDa. Apparently the concentration of BHAL is too low to see a band.
Activity Analysis of BHAL
Initial activity tests of purified fractions
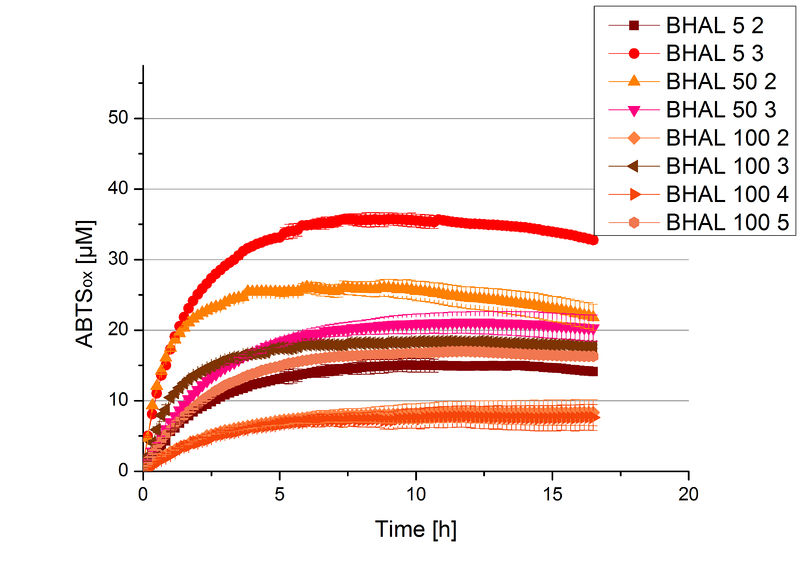
Different fractions of the purification of a cultivation were tested regarding their activity of the produced BHAL. Before and after re-buffering into H2O the protein concentration was determined. The samples were incubated with 0.4 mM CuCl2 for 2 hours. The initial activity tests were done in Britton-Robinson buffer (pH 5) with 0.1 mM ABTS at 25 °C. The change in optical density was measured at 420 nm, reporting the oxidation of ABTS. The protein amount was adjusted in each sample for a comparison. One distinct fraction showed the highest activity: fraction 5% 3 (Fig. 5). The contained laccase amount was calculated by assuming that the most active fraction contains 90 % laccase. This leads to a BHAL concentration of 10,9 ng mL-1.
BHAL activity depending on different ABTS concentrations
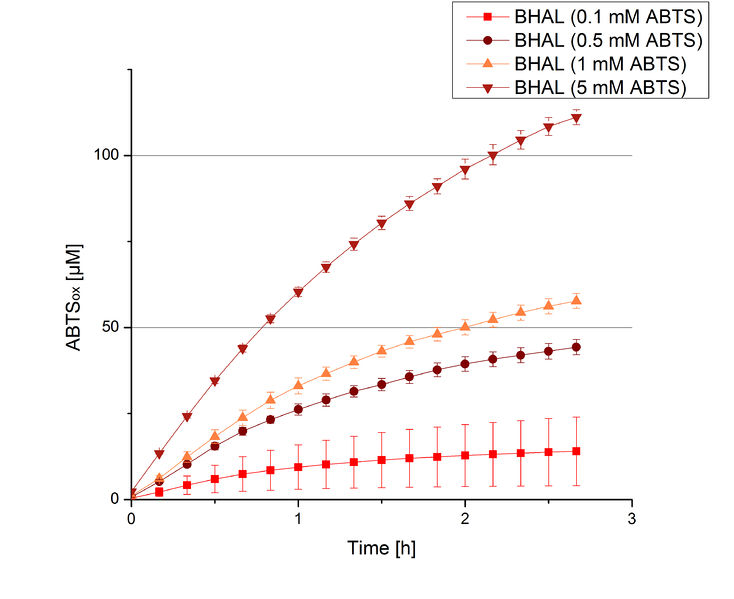
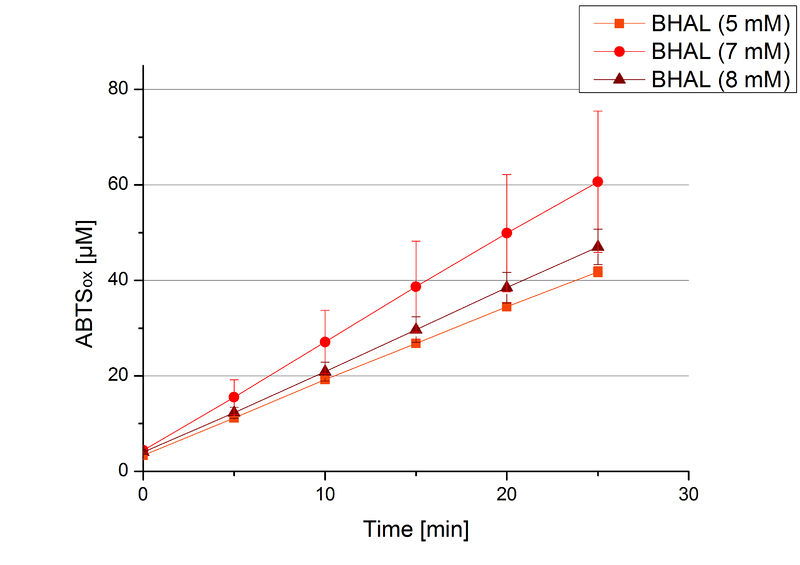
To be able to calculate the activity in Units mg-1, measurements had to be done under substrate saturation. This allows the comparison of Units mg-1 with other laccase activities and data found in literature. For this purpose ABTS concentrations ranging from 0.1 mM to 8 mM were applied in an experimental setup containing Britton-Robinson buffer (pH) and a temperature of 25 °C. For measurements with 0.1 mM to 5 mM ABTS 616 ng BHAL were used (Fig. 6). For measurements with 5 mM to 8 mM ABTS only 308 ng BHAL were applied (Fig. 7). Applying less than 7 mM ABTS a static increase in oxidized ABTS was given. Measurements with 8 mM ABTS showed a slower increase in oxidized ABTS as with 7 mM ABTS (Fig. 7). This may be due to a substrate toxication. The most compromising ABTS concentration was 7 mM with the highest increase in oxidized ABTS. Therefore a substrate saturation was reached with 7 mM ABTS.
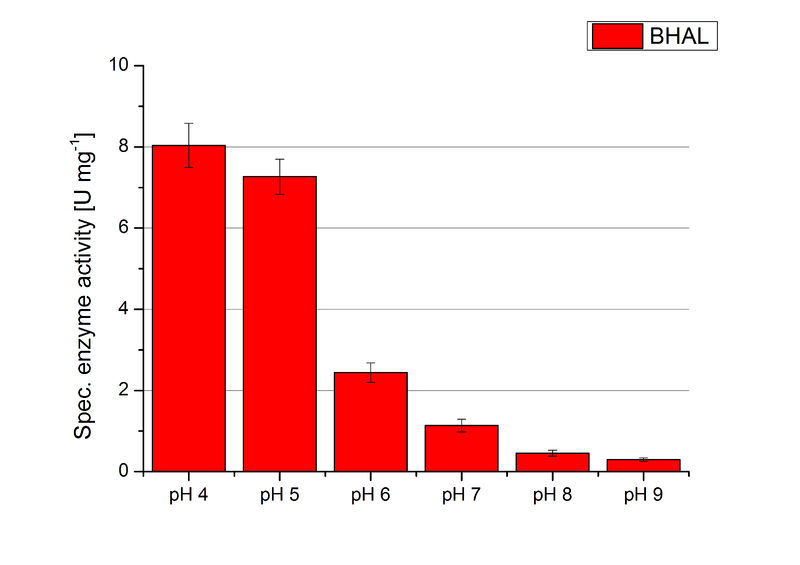
BHAL pH optimum
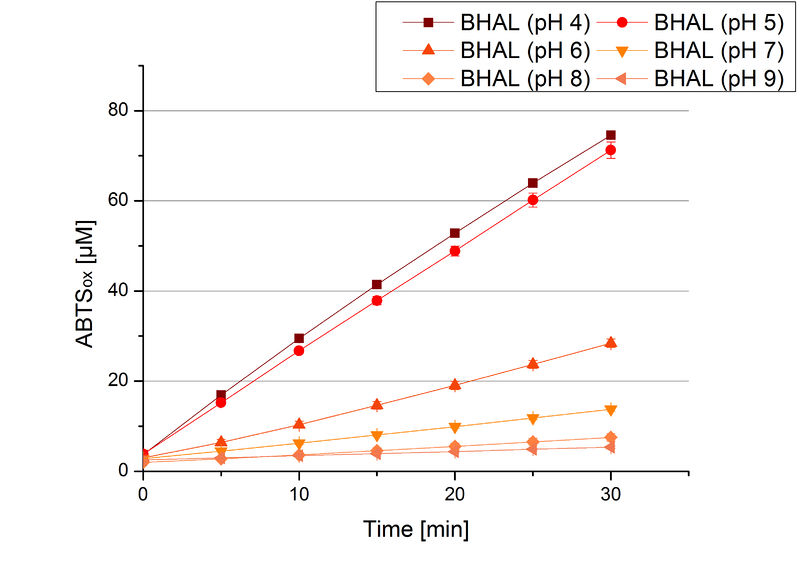
To determine the optimal experimental setup for BHAL activity measurements, the best pH had to be determined. Using Britton-Robinson buffer pHs between pH 4 and pH 9 had been adjusted. 308 ng BHAL per well had been tested under these pH conditions using 7 mM ABTS. The CuCl2 incubated and therefor activated BHAL showed a high activity at pH 4 and pH 5, where most of ABTS was oxidized (compared to Fig. 8 and 9). The calculated specific enzyme activity of BHAL showed high activity at both mentioned pHs (Fig. 10). While BHAL had an activity of ~8 U mg-1 at pH 4 and pH 5, the enzyme activity decreased at higher pHs. At a pH of 6 only 1/3 of enzyme activity could be detected compared to the activity at pH 4 and pH 5. While still active at pH 7, the BHAL is not as suitable as thought for an application at a waste water treatment plant because of its high activity in acidic environments.
BHAL activity at different temperatures
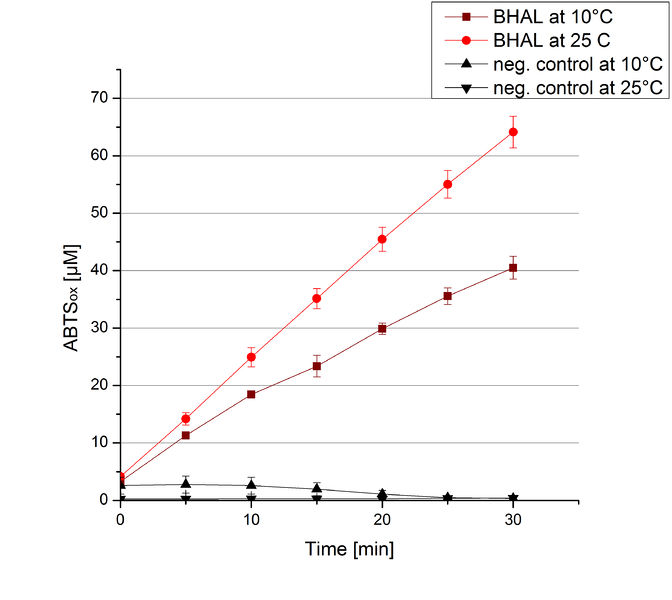
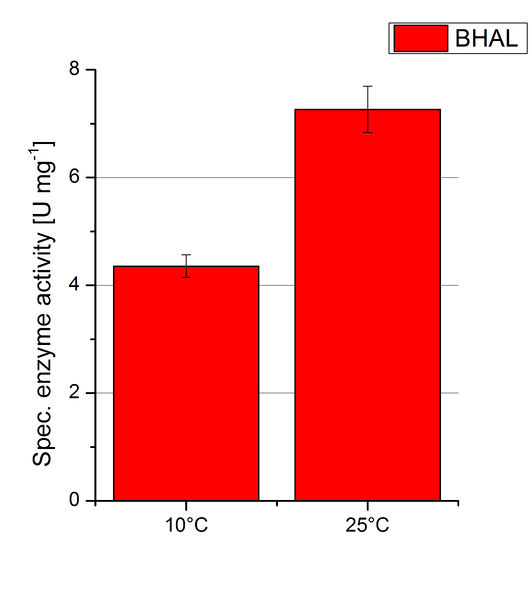
To investigate the activity of BHAL at temperatures that will apply at a waste water treatment plant throughout the year, activity tests were performed at 10 °C and 25 °C as described above. The measurements were conducted for 30 minutes. The obtained results revealed a lower activity of BHAL at 10 °C in comparison to 25 °C (see Fig. 11). The obtained results were used to calculate the specific enzyme activity which was at 4.2 and 7.2 U mg-1, respectively (see Figure 12). The negative control without BHAL but 0.4 mM CuCl2 at 10 °C and 25 °C showed a negligible oxidation of ABTS. The activity of BHAL was increased to about 60 % at 10 °C but nevertheless the observed activity at both conditions was great news for the possible application in waste water treatment plants.
Substrate Analysis
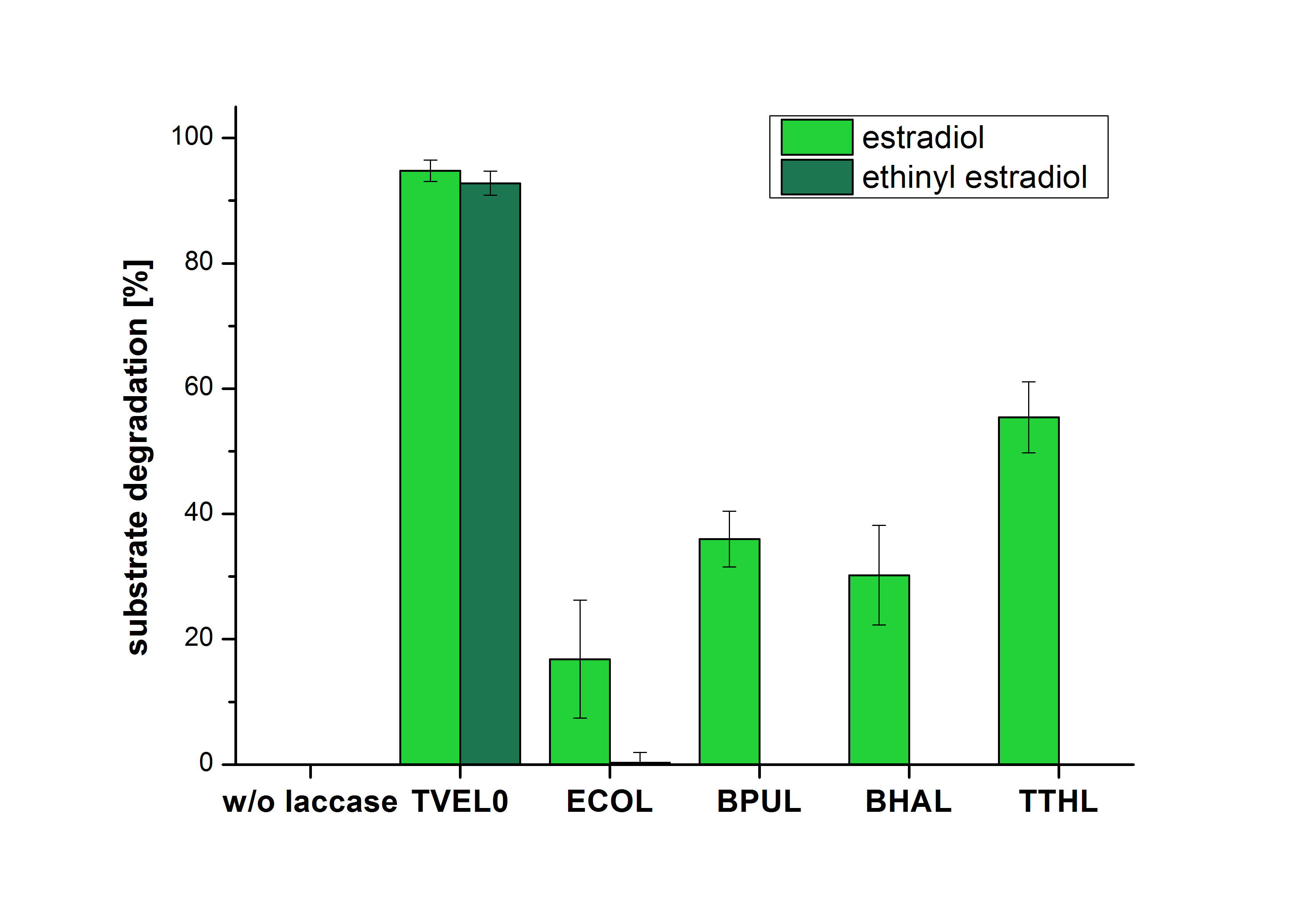
The measurements were made to test if the produced laccases were able to degrade different hormones. Therefore the produced laccases were inserted in the same concentrations (3 µg mL-1) to the different measurement approaches. To work with the correct pH value (which were measured by the Team Activity Test) Britton Robinson buffer at pH 5 was used for all measurements. The initial substrate concentration was 5 µg mL-1. The results of the reactions without ABTS are shown in Figure 2. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are indicated. The X-axis displays the different tested laccases. The degradation was measured at t0 and after five hours of incubation at 30 °C. The negative control was the substrate in Britton Robinson buffer and showed no degradation of the substrates. The bought laccase TVEL0 which is used as positive control is able to degrade 94.7 % estradiol and 92.7 % ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 35.9 % of used estradiol after five hours. ECOL was able to degrade 16.8 % estradiol. BHAL degraded 30.2 % estradiol. The best results were determined with TTHL (laccase from Thermus thermophilus). Here the percentage of degradation amounted 55.4 %.
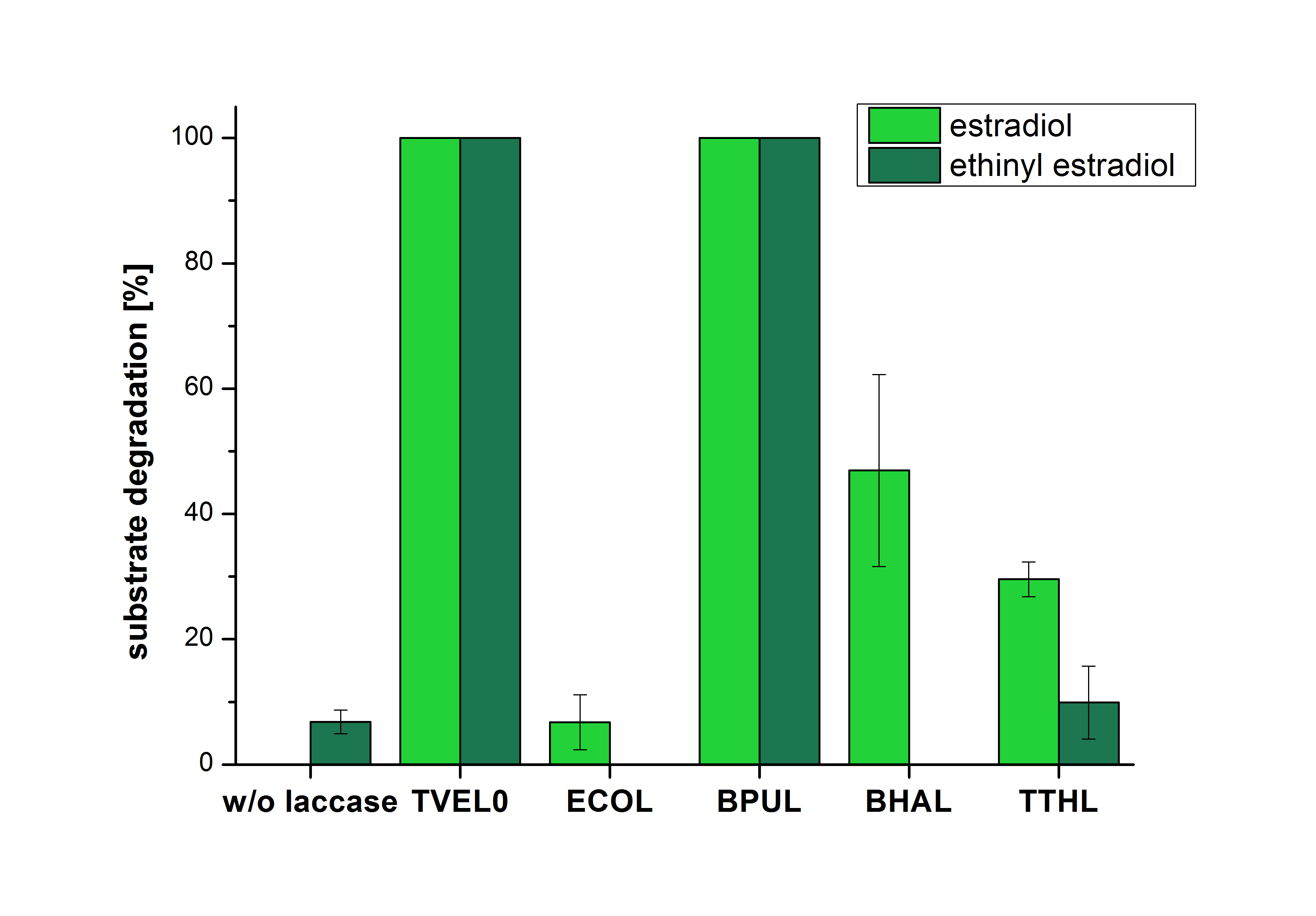
The results of the reactions of the laccases with addition of ABTS are shown in Figure 3. The experimental set ups were the same as the reaction approach without ABTS described above. The X-axis displays the different tested laccases. On the Y-axis the percentages of degraded estradiol (blue) and ethinyl estradiol (red) are shown. The degradation was measured at t0 and after five hours of incubation at 20 °C. The negative control showed no degradation of estradiol. 6.8 % of ethinyl estradiol was decayed. The positive control TVEL0 is able to degrade 100 % estradiol and ethinyl estradiol. The laccase BPUL (from Bacillus pumilus) degraded 46.9 % of used estradiol after ten minutes incubation. ECOL was able to degrade 6.7 % estradiol. BHAL degraded 46.9 % estradiol. With TTHL (laccase from Thermus thermophilus)a degradation 29.5 % were determined.
Immobilization
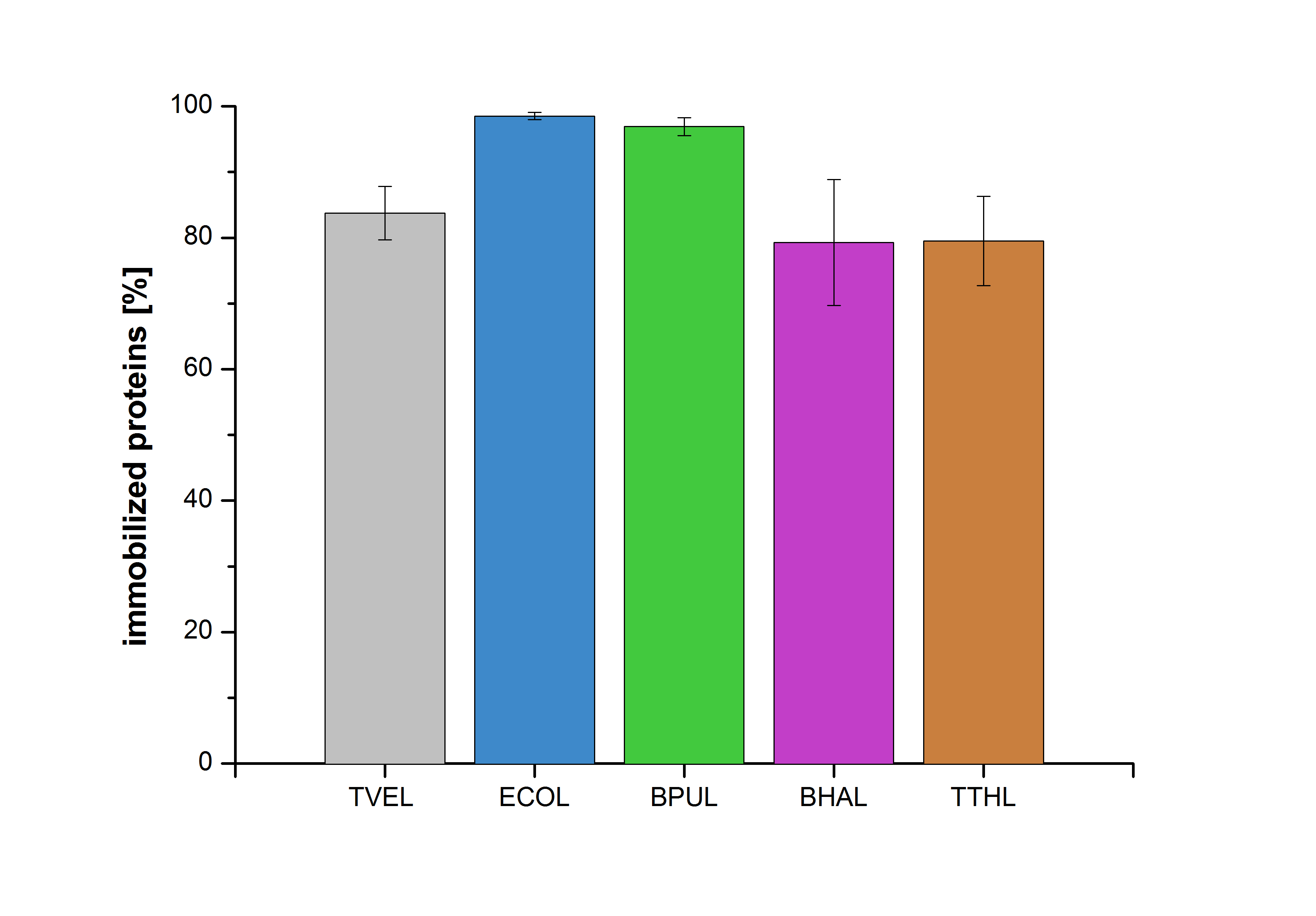
Figure 20 shows the percentage of laccases bound after incubation with CPC-beads, relative to the original concentration. The concentration of laccases in the supernatant after incubation was measured using Roti®-Nanoquant. The results showed that only 21% of BHAL laccases was still present in the supernatant. This illustrates that BHAL was successfully immobilized on the CPC-beads.
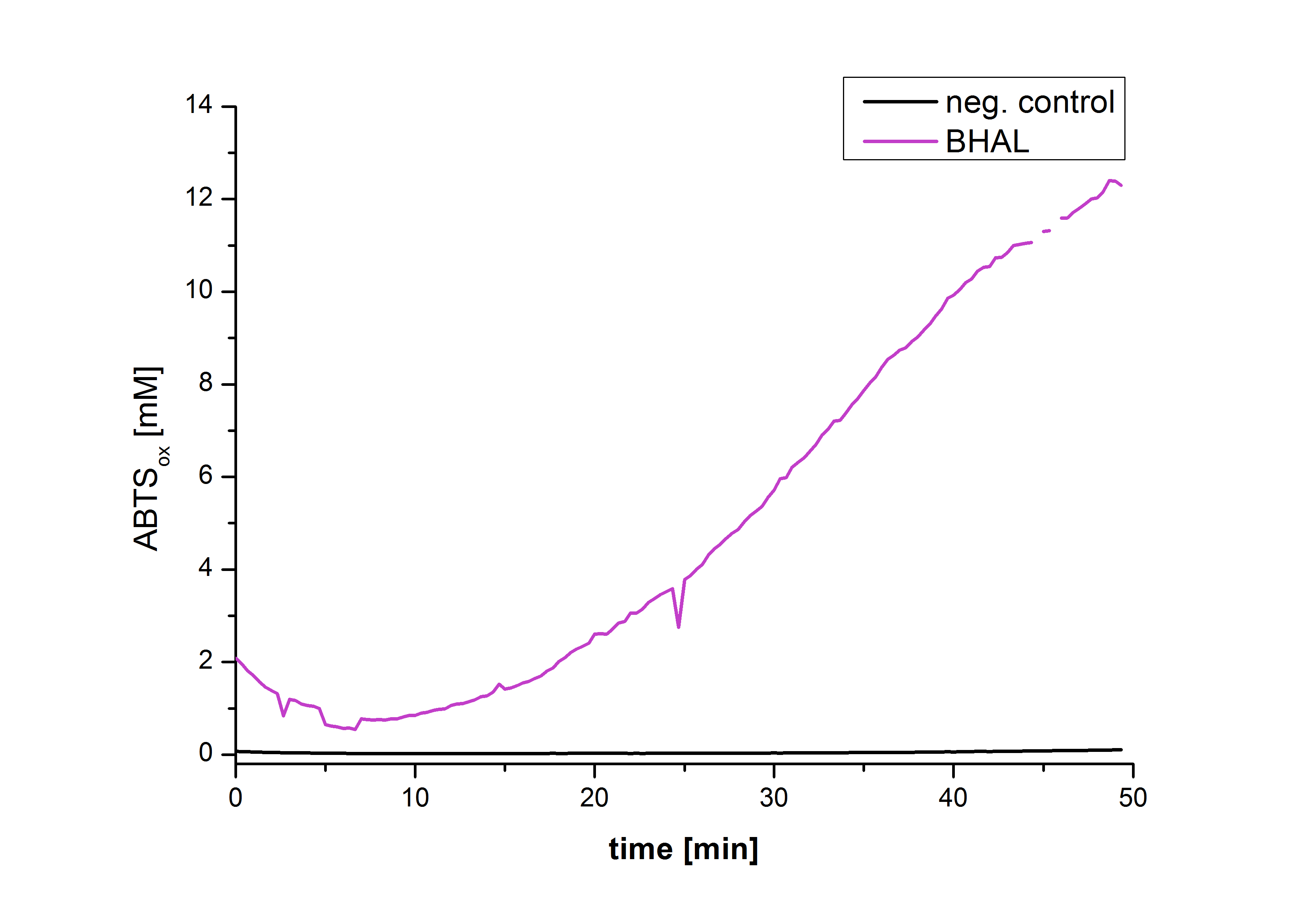
Figure 22 shows the illustration of ABTS oxidation by BHAL with time compared to the negative control. The increase in ABTS oxidized proves laccase activity even if a direct comparison with the original and not immobilized laccase solution was not possible due to the very low concentration of purified BHAL.
| None |