Part:BBa_K863020
bhal laccase from Bacillus halodurans with T7 promoter, RBS and His-tag
bhal laccase from Bacillus halodurans with T7 and HIS
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal XhoI site found at 190
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
First some trials of shaking flask cultivations were made with various parameters to identify the best conditions for production of the His tagged laccase Lbh1 from [http://www.dsmz.de/catalogues/details/culture/DSM-18197.html?tx_dsmzresources_pi5 Bacillus halodurans C-125 ] named BHAL. Due to inactivity of the enzyme in the cell lysate a purification method was established (using Ni-NTA-Histag resin). BHAL could not be detected by SDS-PAGE (theoretical molecular weight of 56 kDa) or activity test by using the BioBrick BBa_K863020 and E. coli KRX as expression system. Due to this results the new BioBrick BBa_K863022 was constructed and expressed E. coli Rossetta-Gami 2. With this expression system the laccase could be produced and analysed via SDS-PAGE. A small scale Ni-NTA-column was used to purify the laccase. The fractionated samples were tested regarding their activity with ABTS and showed ability in oxidizing ABTS. A scale up was not yet performed.
Contents
Cultivation, Purification and SDS-PAGE
Cultivation
The first trials to produce the Lbh1 - laccase from Bacillus halodurans (named BHAL) were performed in shaking flasks with various flask designs (from 100 mL-1 to 1 L flasks, with and without baffles) and under several conditions. The varied parameters in our screening experiments were temperature (27 °C,30 °C and 37 °C), concentration of chloramphenicol (20-170 µg mL-1), induction strategy (autoinduction and manual induction with 0,1 % rhamnose) and cultivation time (6 to 24 h). Furthermore we cultivated with and without 0.25 mM CuCl2 to provide a sufficient amount of copper, which is needed for the active center of the laccase. E.coli KRX was not able to produce active BHAL under the tested conditions, therefore another chassis was chosen. For further cultivations E. coli Rosetta-Gami 2 was transformed with BBa_K863012, because of its ability to translate rare codons. BHAL was produced under the following conditions:
- flask design: shaking flask without baffles
- medium: [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Materials#LB_medium LB]-Medium
- antibiotics: 60 µg mL-1 chloramphenicol and 300 µg mL-1 ampicillin
- temperature: 37 °C
- cultivation time: 24 h
Purification
The cells were harvested and resuspended in Ni-NTA-equilibration buffer, mechanically lysed by sonification and centrifuged. After preparing the cell paste the BHALlaccase could not be purified with the 15 mL column, because of the column was not available. For this reason a small scale purification (6 mL) of the supernatant of the lysate was performed with a [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Production#Syringe_method 1 mL Ni-NTA-column]. The elution was collected in 1 mL fractions.
SDS-PAGE
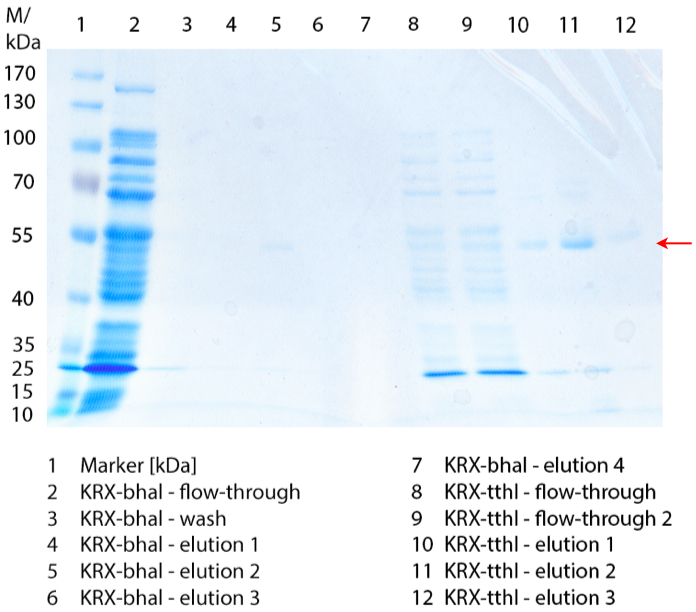
In figure 1 the different fractions of the purified cell lysate of E. coli Rosetta-Gami 2 with BBa_K863022 are shown in a SDS-PAGE. BHAL has a molecular weight of 56 kDa. In lane 5, which corresponds to the elution fraction 2, a faint band of 56 kDa is visible. Therefore the fractions were further analysed by activity test and MALDI-TOF.
Activity Analysis of BHAL
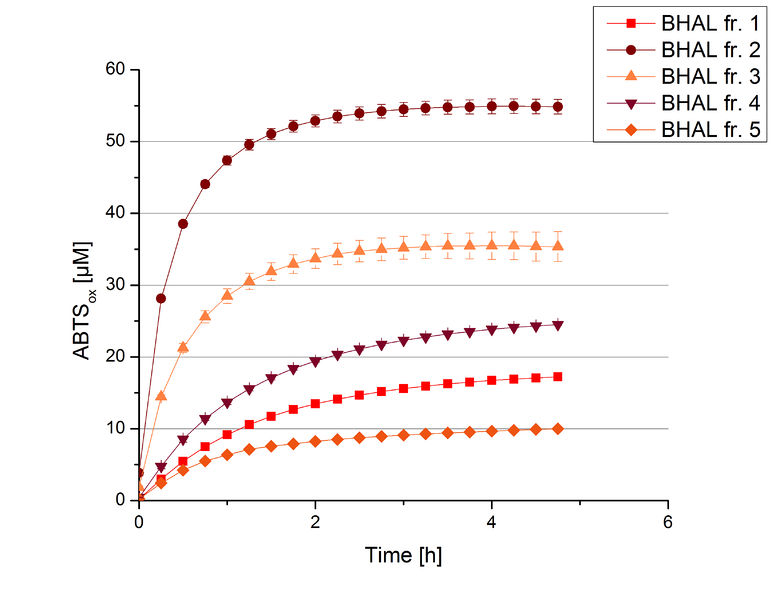
The resulting fractions of the cultivation and purification of BHAL (fraction 1 to 5) were analysed with activity tests. After rebuffering into deionized H2O and incubation with 0.4 mM CuCl2 for 2 hours, the samples were measured with 140 µL sample, 0.1 mM ABTS, 100 mM sodium acetate buffer to a final volume of 200 µL. The change in optical density was measured at 420 nm, reporting the oxidation of ABTS for 5 hours at 25°C. An increase in ABTSox can be seen (Figure 2), indicating produced BHAL laccase in each fraction. Fraction 2 shows the highest amount of ABTSox (55%) reaching saturation after 3 hours. Similar to BPUL laccase, BHAL is capable to reach saturation after 3 hours with approximately oxidizing 55% of the supplied ABTS. Therefore BHAL is going to be characterized further.
//function/degradation
//proteindomain/degradation
| None |
