Part:BBa_K4182007
Lysis-phi X174
Biosafety is an important consideration when designing engineered bacteria. From the beginning, we designed the bacteria on the premise that it would work in the field soil, so we first needed to consider whether our product could be easily controlled for the time of its operation and whether there were potential risks to soil structure, crop growth, and the balance of soil microbiota. So we designed a "suicide system" at the genetic level to ensure that our engineered bacteria would not pose a potential biosecurity risk to the ecological environment. The suicidal behavior of bacteria is a common phenomenon in nature, which is a programmed death mechanism of prokaryotes. quorum sensing (QS) is a form of communication between bacterial cells. Cells synthesize and secrete signal molecules. When the concentration of signal molecules in the environment reaches a certain threshold, a series of genes are activated, and the bacterial population synchronously realizes certain functional and behavioral changes. A quorum-sensing suicide gene circuit has been constructed, and the systematic study and precise regulation of this gene circuit are of great significance both in theory and application. In addition to population-responsive suicide mechanisms, suicide systems with other regulatory modes can also be designed through synthetic biology. Here,we designed a temperature-responsive suicide system to achieve temperature-controlled lysis process by a novel lysis genes (Gene ID: IF654_RS00240).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Profile
Base Pairs
273
Design Notes
The gene was optimized by E. coli codon
Source
Shigella flexneri 2a str. 301 (strain: 301, serotype: 2a)
Usage&Biology
Source and Principle
Biosafety is an important consideration when designing engineered bacteria, and MazF has been commonly used in previously work. Here we develop a novel lysis gene (Gene ID: IF654_RS00240) for the design of controlled suicide circuits for engineering efficiency and biosafety reasons, also can be considered as an alternative of suicide protein MazF.
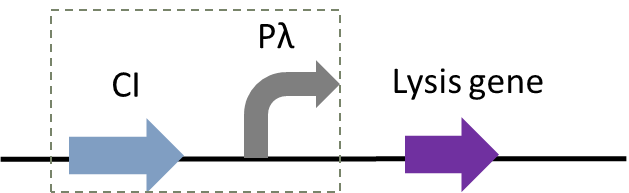
Figure 1: Circuit diagram of plasmid 5: Where CI is the C1857 suppression subsystem, Pλ is the promoter, and the temperature control system is in the dashed box.
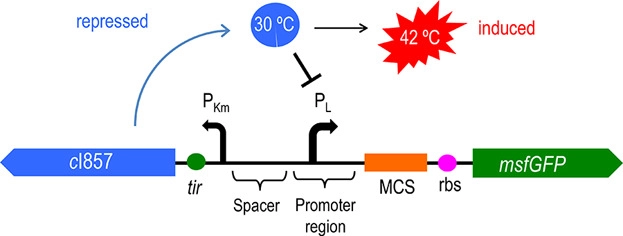
Figure 1 and 2 shows the temperature-controlled suicide circuit and its principle. When bacteria are at a low temperature(30℃), the CI857 protein binds to the Pλ promoter, and downstream lysis gene are unable to be expressed, which will cause the cell growth. While at 42℃, the CI protein will be degraded, leading to the expression of lysis gene and eventually cell death and release of the product
Codon improvement and optimization
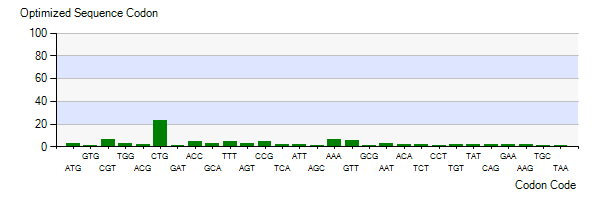
The Lysis gene we used was from Pseudomonas lundensis. To better express the Lysis gene in engineered bacteria, we optimized the codon of the Lysis gene according to the codon preference of Escherichia coli. Figure 5-3 shows the number of codons we optimized to make our codons more in line with Escherichia coli preference.
Figure 3: Codon optimization of plasmid 5
Optimization of Plasmid
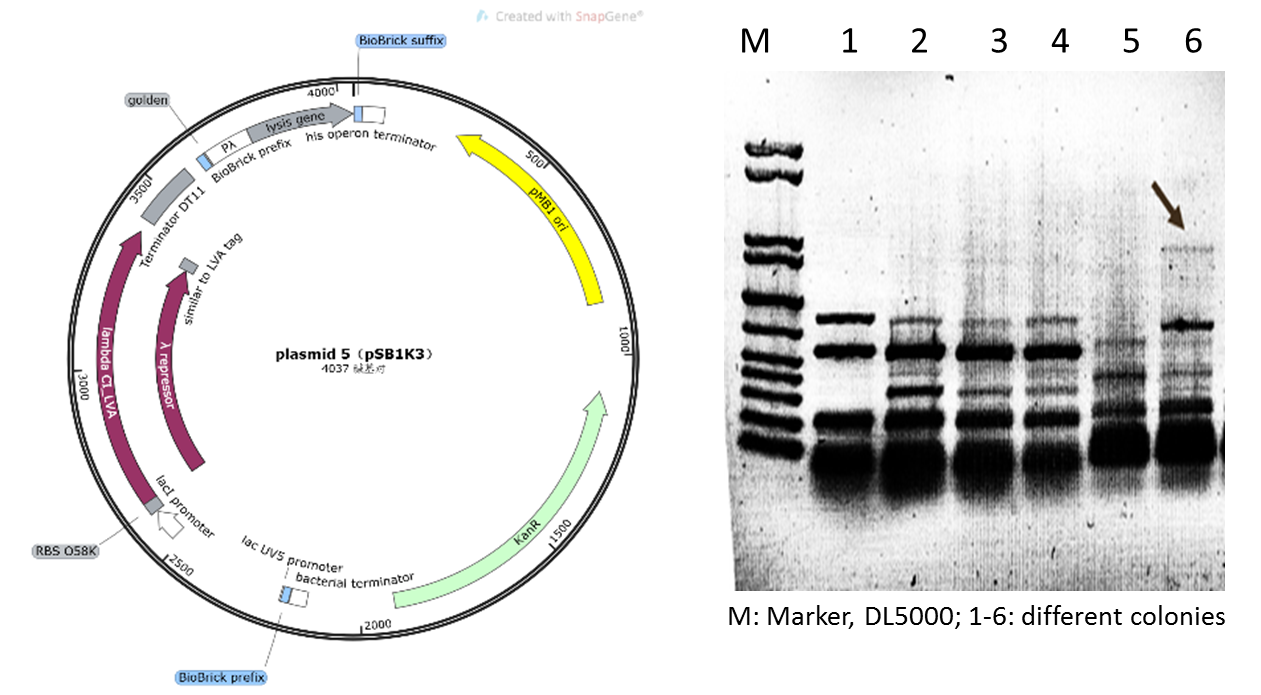
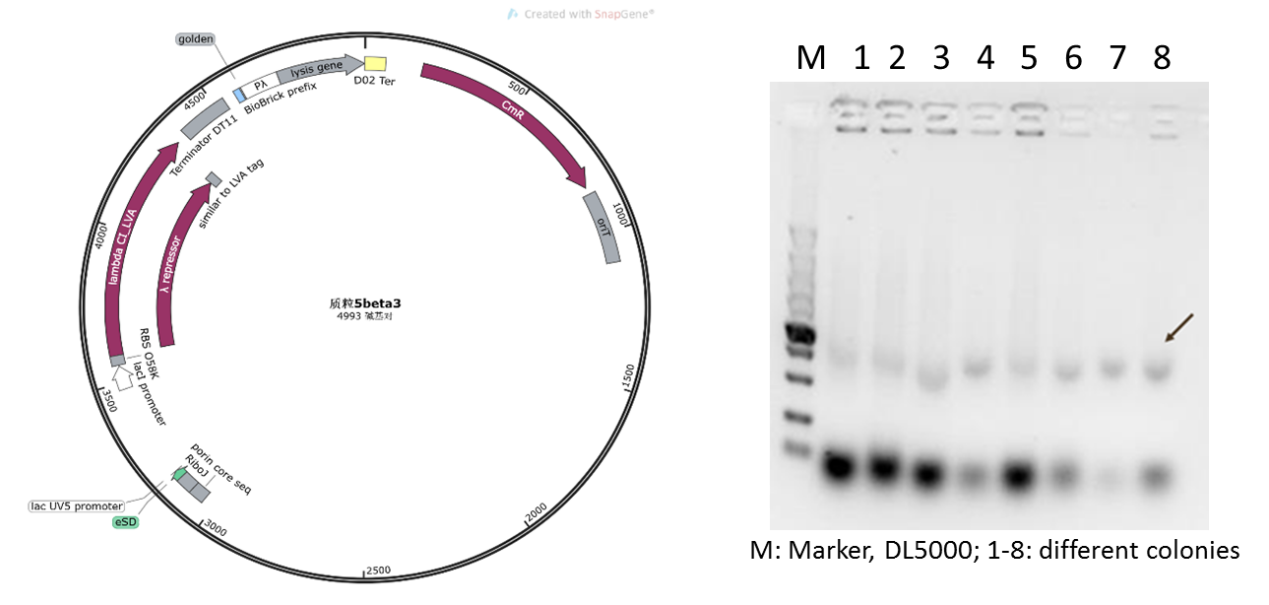
Initially, based on the design of the assay protocol, we planned to construct plasmid 5 using vector backbone pSB1K3. However, in our subsequent experiments, it was found that when the plasmids designed in this way were transferred to DH5α cells after Golden Gate cloning for expression, only dark target bands could be observed in colony PCR (Figure 5), and the extraction of plasmids and sequencing could not be completed due to the low concentration.
Figure 4: Plasmid 5 map based on pSB1K3 and its verification (he target band is approximately 1600bp)
Therefore, we supposed that due to the insufficient copy amount of pSB1K3 plasmid, we could not extract the product with a sufficient concentration in the engineered bacteria. Therefore, we replaced the vector of plasmid 5 with pSEVA341, a higher-copy-number vector and re-constructed the plasmid (Figure 6). As shown in Figure 6, the obvious target bands were observed, and the plasmid was further confirmed by sequencing.
Figure 5: new plasmid 5 with pSEVA341 backbone and its verification
The verification of heat triggered cell lysis and suicide
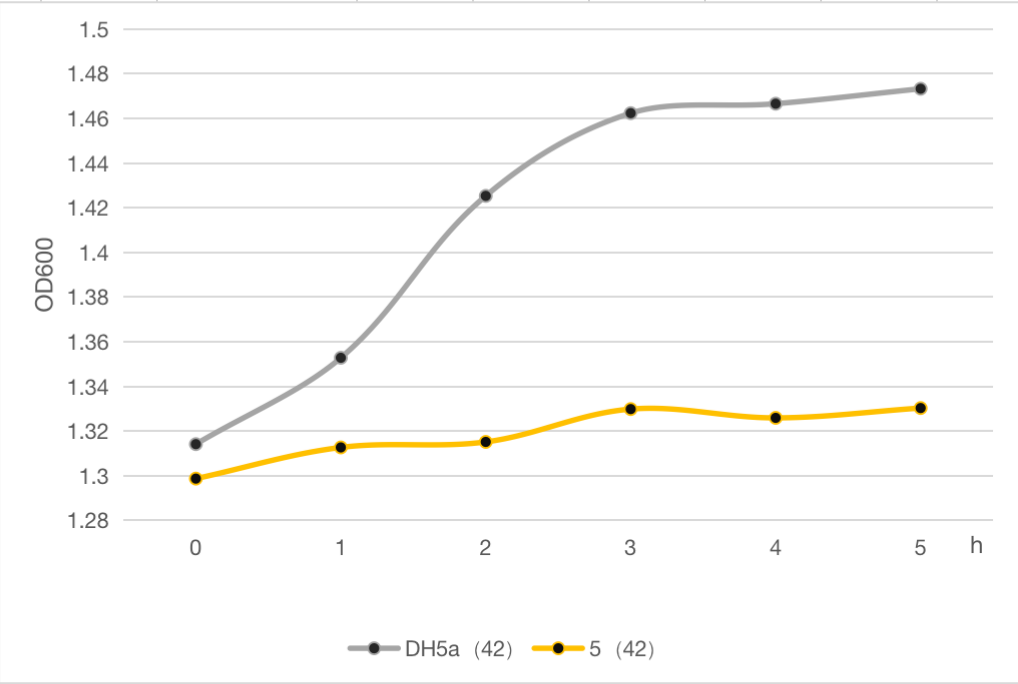
The engineered cell harboring plasmid 5 and blank vector respectively, were culture at 30℃ overnight, and then the temperature was shift to 42℃, while the control group were still cultured at 30℃. The OD600 of each group was detected every 1 h, and the growth curve of these strains were determined as follows.The result clearly demonstrated cell growth was inhibited under high temperature at 42℃, compared to the strain without lysis gene.
Compared to MazF, the lysis protein in our study is effective but shorter and easy to be manipulated, which can be used as an alternative and update for MazF.
Figure 6: The cell growth of strains with suicide plasmid 5 and blank vector
References
1. Din, M.O., et al., Synchronized cycles of bacterial lysis for in vivo delivery. Nature, 2016. 536(7614): p. 81-85.
2. Saeidi, N., et al., Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol Syst Biol, 2011. 7: p. 521.
3. Restrepo-Pineda, S., et al., Thermoinducible expression system for producing recombinant proteins in Escherichia coli: advances and insights. FEMS Microbiol Rev, 2021. 45(6).
4. Aparicio, T., V. de Lorenzo, and E. Martínez-García, Improved Thermotolerance of Genome-Reduced Pseudomonas putida EM42 Enables Effective Functioning of the PL/cI857 System. Biotechnology Journal, 2019. 14(1): p. 1800483.
| None |