Part:BBa_K4365016
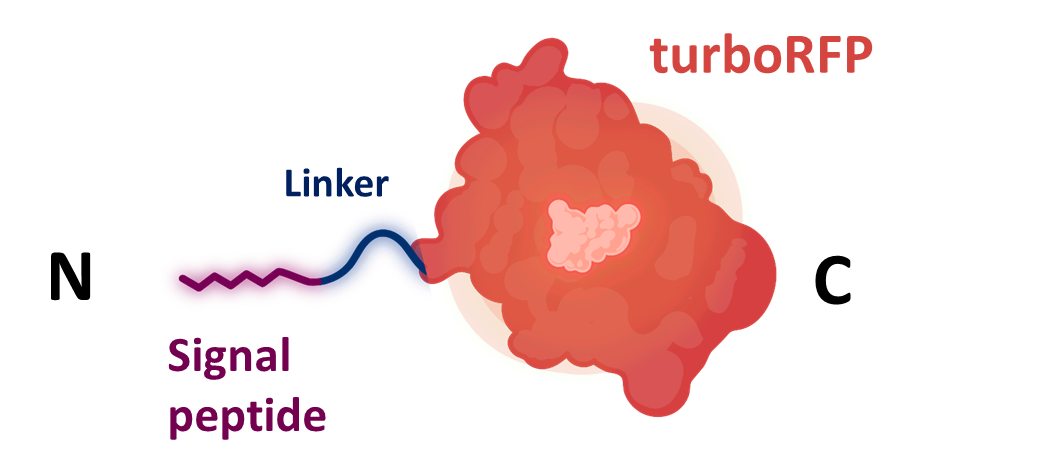
Signal peptide of Aa-PRI2 from Agrocybe aegerita
Aa-PRI2 is a protein found in Agrocybe aegerita and belongs to a family of highly secreted proteins called hydrophobins. The signal peptide from the protein led to turboRFP secretion in Saccharomyces cerevisiae.
| ID | Source | Species | Prediction coefficient [1] | turboRFP secretion | |
|---|---|---|---|---|---|
| sp1 | BBa_K4365006 | MPGI | Magnaporthe grisea | 0.9562 | Yes |
| sp2 | BBa_K4365007 | RodA | Aspergillus nidulans | 0.9553 | Yes |
| sp3 | BBa_K4365008 | HYPI | Aspergillus fumigatus | 0.9703 | No |
| sp4 | BBa_K4365009 | SsgA | Metarhizium anisopliae | 0.9126 | No |
| sp5 | BBa_K4365010 | HCF-4 | Cladosporium fulvum | 0.9126 | Yes |
| sp6 | BBa_K4365025 | HCF-5 | Cladosporium fulvum | 0.9234 | NA |
| sp7 | BBa_K4365026 | CCG-2 | Neurospora crassa | 0.9566 | NA |
| sp8 | BBa_K4365011 | HCF-1 | Cladosporium fulvum | 0.6850 | Yes |
| sp9 | BBa_K4365027 | HCF-2 | Cladosporium fulvum | 0.8144 | NA |
| sp10 | BBa_K4365012 | HCF-3 | Cladosporium fulvum | 0.7934 | No |
| sp11 | BBa_K4365013 | SC3 | Schizophyllum commune | 0.6327 | No |
| sp12 | BBa_K4365014 | ABH3 | Agaricus bisporus | 0.9458 | Yes |
| sp13 | BBa_K4365028 | COH1 | Coprinus cinereus | 0.8545 | NA |
| sp14 | BBa_K4365015 | FBH1 | Pleurotus ostreatus | 0.3367 | Yes |
| sp15 | BBa_K4365016 | Aa-PRI2 | Agrocybe aegerita | 0.6356 | Yes |
| sp16 | BBa_K4365033 | Hyd-Pt1 | Pisolithus tinctorius | 0.8352 | NA |
| sp17 | BBa_K4365017 | HFBI | Trichoderma reesei | 0.9851 | Yes |
| sp18 | BBa_K4365018 | HFBII | Trichoderma reesei | 0.6862 | Yes |
| sp19 | BBa_K4365019 | α-mating factor | Saccharomyces cerevisiae | 0.9988 | Yes |
- ↑ José Juan Almagro Armenteros et al. (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks Nature Biotechnology, 37, 420-423, doi: 10.1038/s41587-019-0036-z
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Usage and Biology
Secretion signal peptides can be fused to a protein to direct it through the secretory pathway out of the cell [1]. Such secretion of recombinant proteins facilitates the protein purification process and the removal of GMOs [2]. However, commonly used signal sequences like the S. cerevisiae alpha-factor prepro-peptide K4365019 and the HSP150, are very long [2][3] which makes their use in genetic constructs limited. K4365016 as a part of the collection of signal peptides represents a shorter sequence that could be inserted into the gene of interest simply by PCR and could display higher secretion efficiency.
The sequences of the hydrophobic signal peptide was collected from literature [4] and was extracted via analysis of their sequence using the SignalP - 5.0 signal peptide predictor tool [5].
Identified sequence was codon-optimized for S. cerevisiae using the codon optimization tool on GenScript. Then it was synthesized by IDT as a gBlock together with a flexible glycine linker (K4365005) and the turboRFP gene (K4365020) [6].
Hydrophobins
Hydrophobins are a group of small (~100 amino acids) proteins that are expressed in ascomycetes and basidiomycetes, which are very efficiently secreted [2].
After secretion from the fungus, they can assemble on the cell walls to form a hydrophobic coating. For this reason, the functions of hydrophobins are related to cell surface activity. For example, they can cover hyphae so the surface tension can be lowered and the hyphae can breach the water/air interface, thus escaping from the aqueous environment and growing into the aerial hyphae [7]. They also mediate the adhesion of the hyphae to hydrophobic surfaces such as in the case of pathogen-host interaction or symbiosis, as observed in lichens and in ectomycorrhizae (the symbiotic relationship between a fungal symbiont and the roots of a plant) [7]. Hydrophobins also form a hydrophobic sheath to protect the surface of conidia, spores, and caps of fungi against wetting and desiccation [7].
Signal peptide secretion screening
Signal peptide siquence was cloned together with turboRFP K4365020 into the level 1 shuttle plasmid K4365022 (Figure 1).
After the transformation with the shuttle plasmid, the yeast colonies were grown on W0 medium plates supplemented with histidine, leucine, and methionine and were used for secretion assays in flasks and in a 48-well plate. The α-mating factor pre-pro signal peptide K4365019 was used as a positive control as it has been shown to be able to secrete proteins [8]. A turboRFP without signal peptide was employed as a negative control to account for the amount of free turboRFP present in the media due to cell lysis.
Secretion measurement protocols:
The first secretion experiment was conducted in flasks and showed (Figure 2A) that cell lysis was low as indicated by the low fluorescence of the negative control. In order to have consistent results and ensure confidence we set up biological triplicates. For this, turboRFP secretion was measured using 48-well plate approach. Cells with induced expression were pipetted into the wells of the plate and grown overnight. Starting at 1 hour after induction, samples were taken and the growth rate was monitored by measuring turbidity (NTU) and the development of fluorescence in the supernatant was measured on a plate reader.
K4365016 showed turboRFP secretion in both flask and 48-well plate measurement approaches (Figure 2).

References
- ↑ Alberts, B. (2015) Molecular Biology of the Cell. 6th Edition, Garland Science, Taylor and Francis Group, New York.
- ↑ 2.0 2.1 2.2 Kirsten Kottmeier, Kai Ostermann, Thomas Bley, Gerhard Rödel (2011) Hydrophobin signal sequence mediates efficient secretion of recombinant proteins in Pichia pastoris, Appl Microbiol Biotechnol 91, pages133–141, https://doi.org/10.1007/s00253-011-3246-y
- ↑ Russo P, Kalkkinen N, Sareneva H, Paakkola J, Makarow M. A heat shock gene from Saccharomyces cerevisiae encoding a secretory glycoprotein. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3671-5. doi: 10.1073/pnas.89.9.3671. Erratum in: Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8857. PMID: 1570286; PMCID: PMC525552.
- ↑ Denis Tagu, Birgit Nasse, Francis Martin (1996) Cloning and characterization of hydrophobins-encoding cDNAs from the ectomycorrhizal Basidiomycete Pisolithus tinctorius, Gene Volume 168, Issue 1, Pages 93-97 https://doi.org/10.1016/0378-1119(95)00725-3
- ↑ José Juan Almagro Armenteros et al. (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks Nature Biotechnology, 37, 420-423, doi: 10.1038/s41587-019-0036-z
- ↑ Merzlyak, E., Goedhart, J., Shcherbo, D. et al. (2007) Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat Methods 4, 555–557 (2007). https://doi.org/10.1038/nmeth1062
- ↑ 7.0 7.1 7.2 Khalesi, M., Gebruers, K. & Derdelinckx, G. (2015) Recent Advances in Fungal Hydrophobin Towards Using in Industry, Protein J, 34, 243–255 . https://doi.org/10.1007/s10930-015-9621-2
- ↑ 8.0 8.1 Peñas M. M. et al. (1998) Identification, characterization, and In situ detection of a fruit-body-specific hydrophobin of Pleurotus ostreatus, Appl Environ Microbiol. 64(10):4028-34. doi: 10.1128/AEM.64.10.4028-4034.1998. PMID: 9758836; PMCID: PMC106595.
| None |