Part:BBa_K3606059
P3 driven mcbABCD and PtetR driven mcbEFG
We used McbA-D to inhibit the growth of other bacteria in human intestine so as to enhance Nissle's competitiveness,as well as to reduce the risk of illness caused by some opportunistic pathogens. We introduced McbE-G to inhibit the growth of other bacteria in human intestine so as to enhance Nissle's competitiveness,as well as to reduce risk of illness caused by some opportunistic pathogens.At the same time, we put McbE-G under the control of pTetR in order to response to AHL-LuxR.
Background:
We used McbA-D to inhibit the growth of other bacteria in human intestine so as to enhance Nissle's competitiveness,as well as to reduce the risk of illness caused by some opportunistic pathogens. We introduced McbE-G to inhibit the growth of other bacteria in human intestine so as to enhance Nissle's competitiveness,as well as to reduce risk of illness caused by some opportunistic pathogens.At the same time, we put McbE-G under the control of pTetR in order to response to AHL-LuxR.
Design:
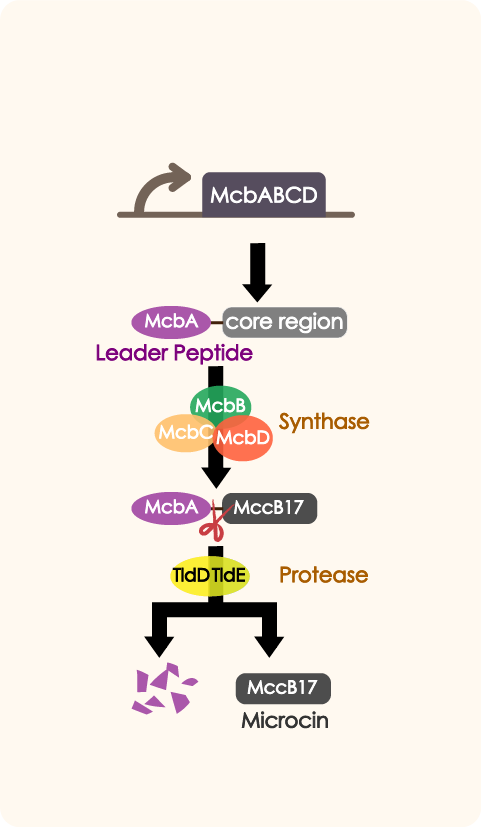
Here, we tried to improve the former antimicrobial peptide(mccb17) expressing system of 2019 Fudan BBa_K3245010. By dividing into the peptide expressing parts and the immunity parts, we wanted to manipulate their expression level with more efficiency.
Usage and Biology:
We introduced mcbABCD to our improved antibiotic expressing system to inhibit the growth of other bacteria in human intestine so as to enhance Nissle's competitiveness, as well as to reduce risk of illness caused by some opportunistic pathogens.Driven by a series of constitutive promoters with different strength which include P3, mcbABCD is expressed in E. coli in a controlled, reliable manner.
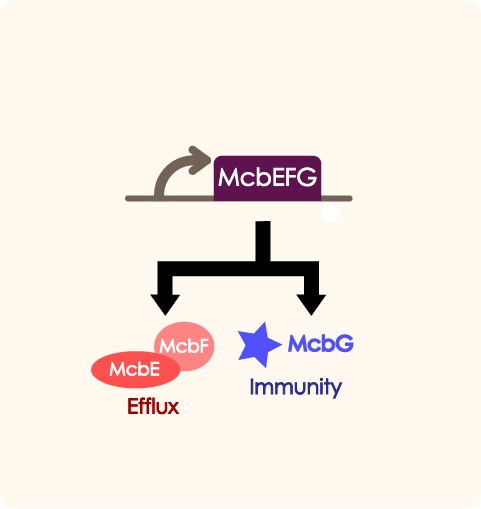
McbEFG works as an adjusting part in quorum sensing system. When the number of engineered bacteria is low, the part will help export antibiotic and provide self immunity in order to help proliferate. PtetR determines the expression of mcbEFG when the number of bacteria is at different level.
Sequence and Features
- 10INCOMPATIBLE WITH RFC[10]Illegal EcoRI site found at 5559
Illegal XbaI site found at 5544
Illegal PstI site found at 2382
Illegal PstI site found at 2415 - 12INCOMPATIBLE WITH RFC[12]Illegal EcoRI site found at 5559
Illegal PstI site found at 2382
Illegal PstI site found at 2415
Illegal NotI site found at 5551 - 21INCOMPATIBLE WITH RFC[21]Illegal EcoRI site found at 5559
Illegal BamHI site found at 3378 - 23INCOMPATIBLE WITH RFC[23]Illegal EcoRI site found at 5559
Illegal XbaI site found at 5544
Illegal PstI site found at 2382
Illegal PstI site found at 2415 - 25INCOMPATIBLE WITH RFC[25]Illegal EcoRI site found at 5559
Illegal XbaI site found at 5544
Illegal PstI site found at 2382
Illegal PstI site found at 2415
Illegal NgoMIV site found at 2044
Illegal AgeI site found at 2216 - 1000COMPATIBLE WITH RFC[1000]
Method:
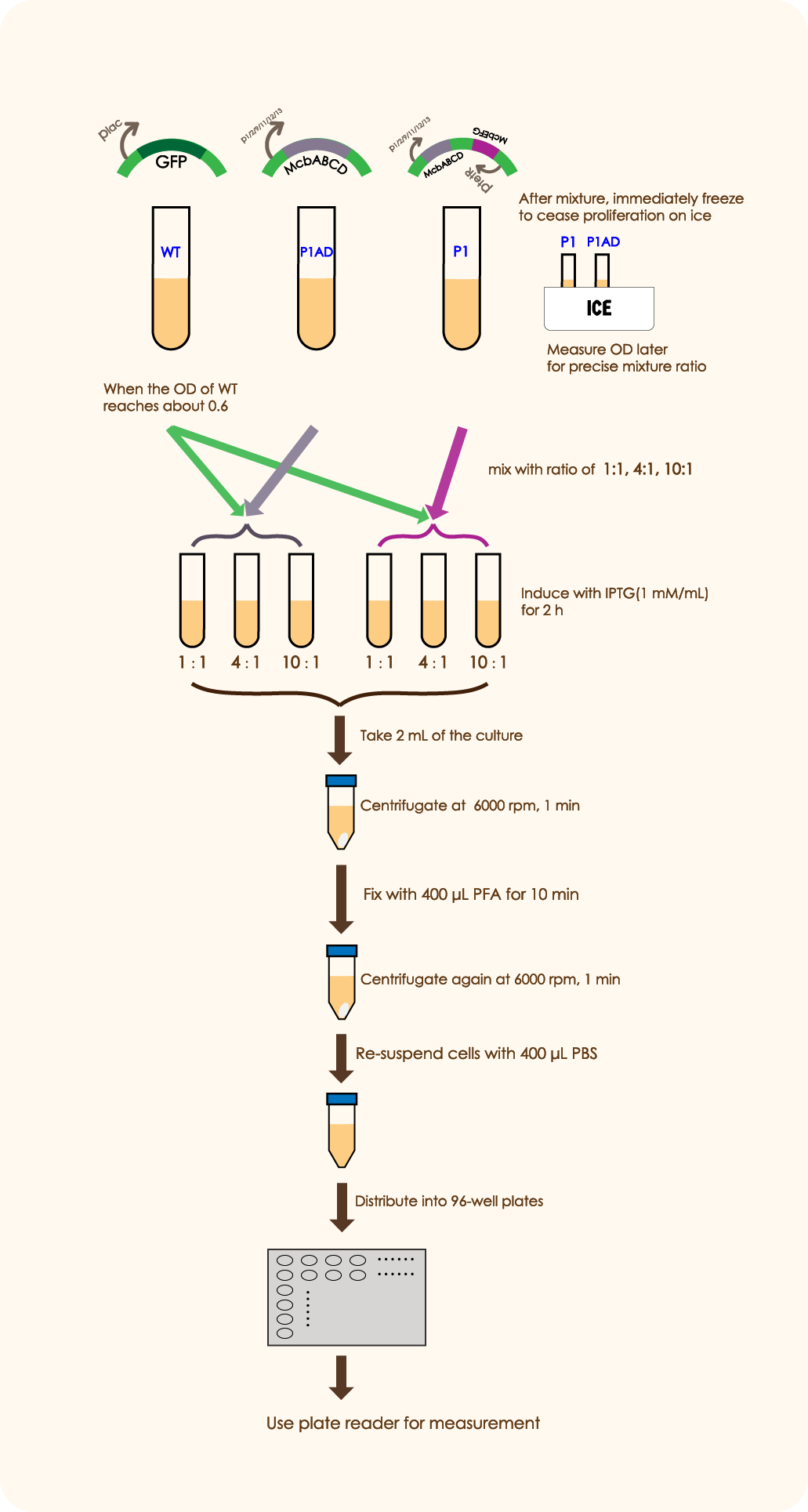
In order to prove that McbABCDEFG does have an effective antibacterial effect as well as McbEFG can protect the engineered bacteria themselves and help the secretion of antimicrobial peptides more effectively, we designed the following experiment:
We mixed WT E.coli (expressing GFP driven by plac) and E.coli with mcbABCD-mcbEFG-ptetR in different ratios (5:7 20:7 50:7), and measured the OD value of the bacteria two hours after adding an inducer, which can be used to reflect the antibacterial effect of antimicrobial peptides. We followed the same method as above to mix WT E. coli and E.coli with merely mcbABCD, induce and measure the OD value.
Results:
We constructed the plasmid and successfully expressed the system, here is the electrophoresis map.
We could see an overall decrease of GFP expression in nearly all groups cocultured with P3 or P3AD than the control group which only contains WT, indicating that the antimicrobial peptide(MccB17) encoded by mcbABCD does have a negative effect on over microbial. In this case, the living status of WT cells and its function in creating GFP is strongly restricted by the toxic environment, proving that our part mcbABCD has worked successfully.
While inside each paired group, there clearly are a better inhibition effect in P3 group than P3AD group, as the MEFL/particle is much lower in the P3 group than the P3AD group when driven by certain promoters. This shows that the immunity function of mcbEFG is working successfully, as the E.coli expressing mcbEFG can help the engineered strain to survive with efflux exporting the toxic peptide, as well as better killing off other strains to gain survival advantage.
It is worth noting that when the mixing ratio of WT E. coli and McbABCDEFG-expressing bacteria is 50:7, the OD values of each group are very close to those of the control group (only induced WT E. coli). This shows that when the concentration of the engineered bacteria is very low, the antimicrobial peptides are difficult to exert antibacterial effect and the engineered bacteria have no competitive advantage.
Further Application:
For futher application, this part expresses antimicrobial peptide(mccb17) as well as providing immunity to create survival advantage for the engineered strain. These part are especially useful to be expressed in vivo because research has proved that it can also ease the inflammation in intestine by limiting the expansion of related pathogens and pathobionts.
References:
Collin F, Maxwell A. The Microbial Toxin Microcin B17: Prospects for the Development of New Antibacterial Agents. J Mol Biol. 2019;431(18):3400–3426. doi:10.1016/j.jmb.2019.05.050
S. Duquesne, D. Destoumieux-Garzón, J. Peduzzi, S. Rebuffat. Microcins, gene-encoded antibacterial peptides from enterobacteria
Sassone-Corsi M, Nuccio SP, Liu H, Hernandez D, Vu CT, Takahashi AA, Edwards RA, Raffatellu M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016 Dec 8;540(7632):280-283.
Mutalik VK, Guimaraes JC, Cambray G, Lam C, Christoffersen MJ, Mai QA, Tran AB, Paull M, Keasling JD, Arkin AP, Endy D. Precise and reliable gene expression via standard transcription and translation initiation elements. Nat Methods. 2013 Apr;10(4):354-60. doi: 10.1038/nmeth.2404. Epub 2013 Mar 10. PMID: 23474465.
| None |