Part:BBa_K1150000:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1150000
User Reviews
UNIQ535cdb31c9371e74-partinfo-00000000-QINU UNIQ535cdb31c9371e74-partinfo-00000001-QINU UNIQ535cdb31c9371e74-partinfo-00000002-QINU
|
•
Peking 2020 |
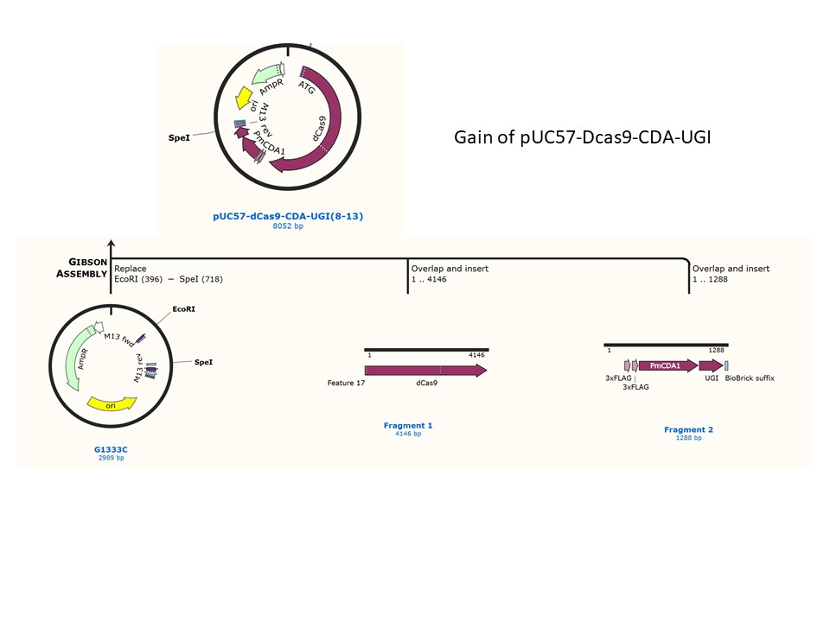
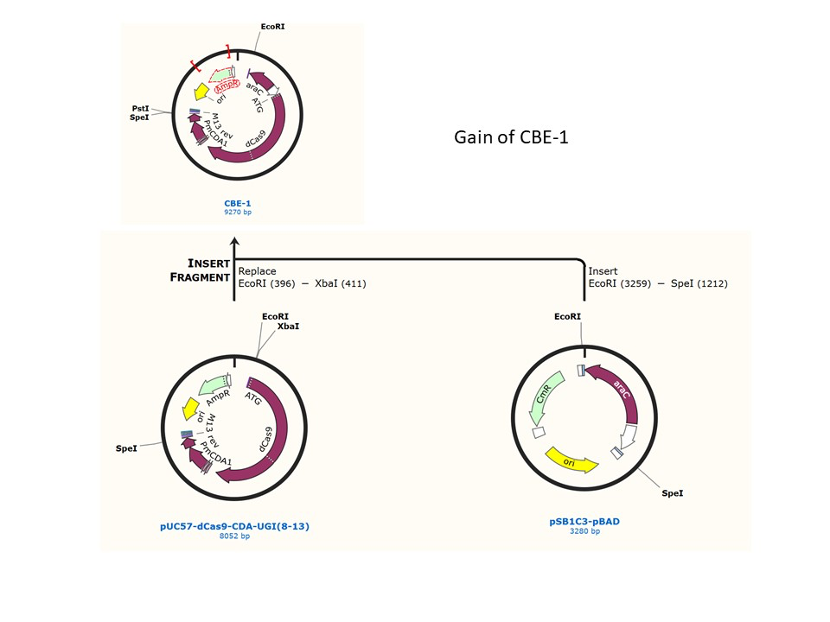
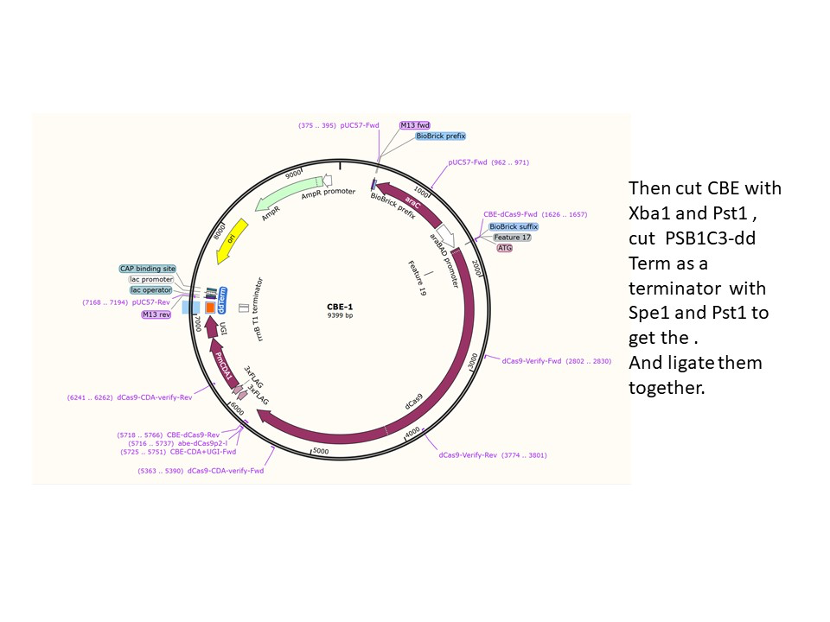
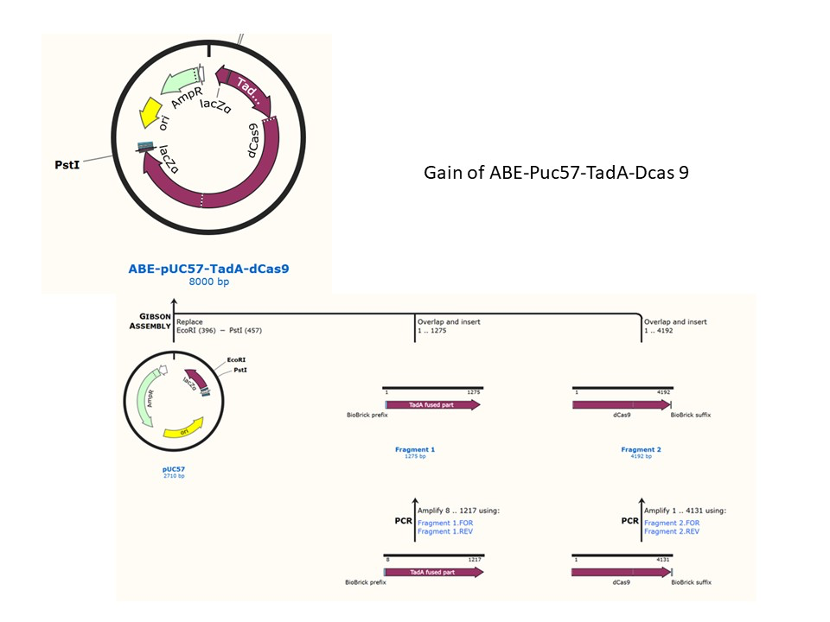
We designed construction of two CRISPR system guided base editor, BBa_K3645008 ABE (A → G on binding site), BBa_K3645011 CBE (C → T on binding site), see below for the fusion protein overview.
David Liu’s lab created the first base editor in 2016 (Komor et al., 2016) and since then has been trying to expand their precision editing capabilities. Base editors make specific DNA base changes and consist of a catalytically impaired Cas protein (dCas or Cas nickase) fused to a DNA-modifying enzyme, in this case a deaminase. Base changes from C•G-to-T•A are mediated by cytosine base editors (CBEs) and base changes from A•T-to-G•C are mediated by adenine base editors (ABEs). How does this work? Through molecular biology teamwork. The guide RNA (gRNA) specifies the editing target site on the DNA, the Cas domain directs the modifying enzyme to the target site, and the deaminase induces the DNA base change without a DNA double-strand break. But base editors aren’t perfect. They may be slow, can only target certain sites, or make only a subset of base substitutions. (addgene blog by Susanna Bachle) We used the existing plasmids for enzyme digestion and ligation, and ePCR was added to the BioBrick connector. After multiple rounds of splicing and assembly, we obtained the ABE and CBE we needed. The schematic diagrams are as follows: CBEUntil 2016, precise single base changes were only possible through exploiting the homology-directed repair (HDR) pathway which occurs in cells at low frequencies and relies on the efficient delivery of donor DNA to facilitate repair. Since the development of CRISPR-mediated base editing (BE), these types of repairs can now be done more efficiently than before. A base editor precisely changes a single base with an efficiency typically ranging from 2575%, while the success of precise change via HDR limited to 0-5%. This blog post covers a brief review of different basic BE technologies and their adaptation for plant genome editing. (addgene blog by Guest Blogger) In 2016, two independent groups (komor et al., 2016 and Nishida et al., 2016) invented CRISPR base editor by linking cytosine deaminase with cas9 cleavage enzyme (ncas9), thus achieving accurate and efficient base rewriting in the genome. Ncas9 creates a gap in DNA by cutting only one single strand, thus greatly reducing the possibility of harmful insertion deletion. After binding with DNA, CBE deamination of target cytosine (C) into uracil (U) base. Later, the resulting U•G pairs were repaired through the cell mismatch repair mechanism to convert the original C•G pair into T•A, or reduced to the original C•G through the uracil glycosylase mediated base excision repair. The presence of UGI minimizes the second result and increases the production of required T•A base pairs. 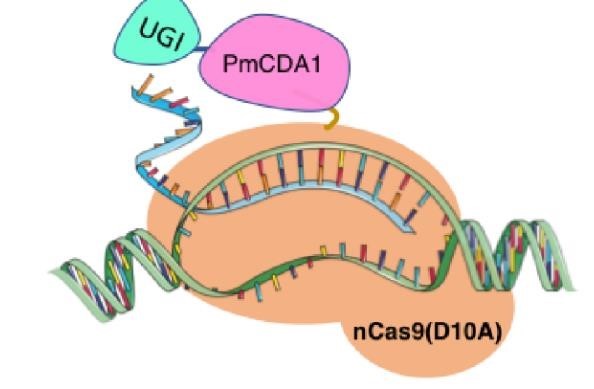
(addgene blog by Guest Blogger)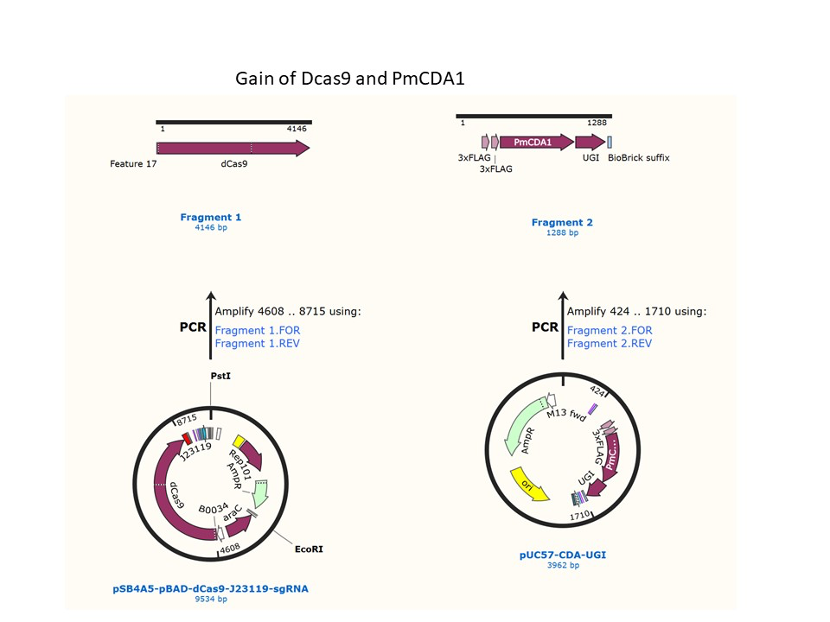
ABE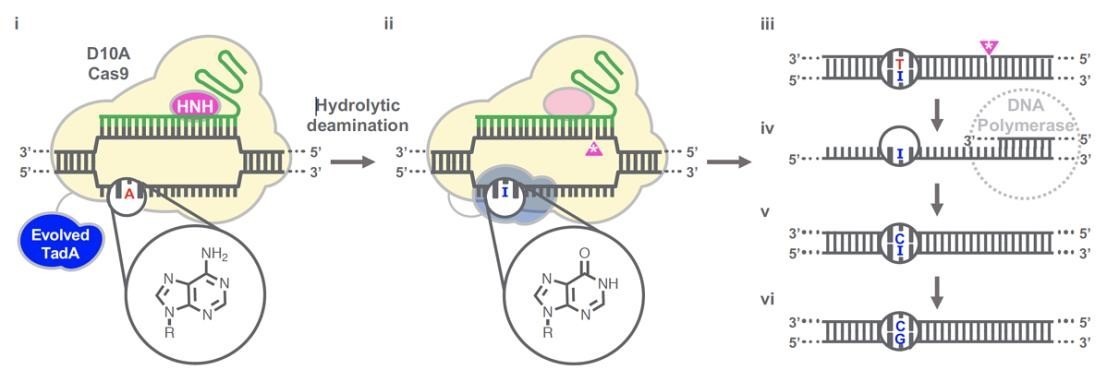
(Gaudelli et al., 2020.)Gaudelli et al. have successfully developed an adenosine deaminase, which can act on DNA for adenine base editing. They first created a defective chloramphenicol resistance gene (CamR) by introducing a point mutation (H193Y). Reversal of this mutation by adenine base editor will restore antibiotic resistance. To find such a protein, they created a mutant library of E.coli tRNA adenosine deaminase (ecTadA), fused it with dcas9, and transformed it into E.coli containing the defective CamR gene. Screening of viable colonies and subsequent rounds of evolution and engineering produced a mutant TadA (TadA *), which accepted DNA as a substrate satisfactorily. The artificially evolved adenosine deaminase catalyzes the transformation of target "A" into "I" (inosine), which is regarded as "G" by cell polymerase. Subsequently, a primitive genome A•T base pair was transformed into a G•C base pair. Since inosine excision repair is not as active as uracil excision, ABE does not require any additional inhibitor proteins, such as UGI in CBE. 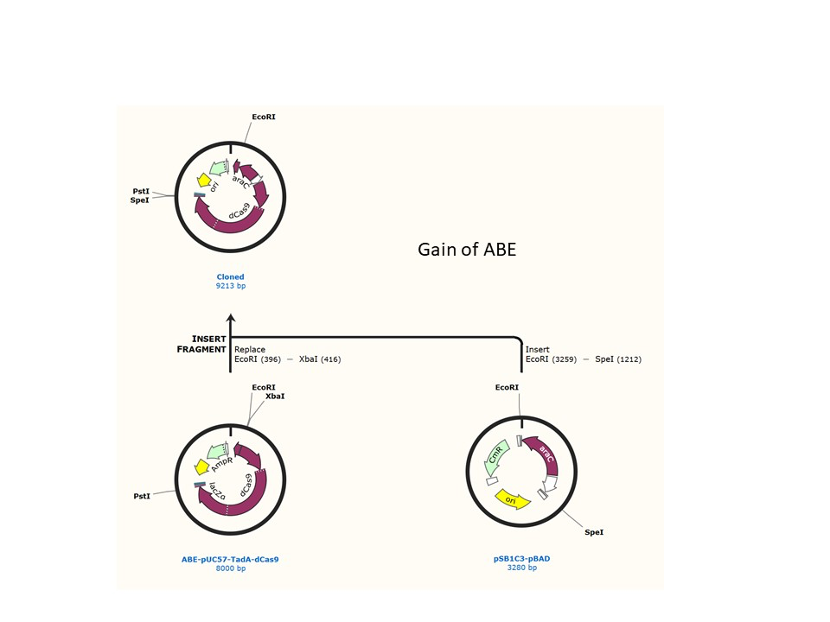
Citation[1] Madej T, Lanczycki CJ, Zhang D, Thiessen PA, Geer RC, Marchler-Bauer A, Bryant SH. " MMDB and VAST+: tracking structural similarities between macromolecular complexes. Nucleic Acids Res. 2014 Jan; 42(Database issue):D297-303 [2] Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature. 2017 Nov 23;551(7681):464-471. doi: 10.1038/nature24644. Epub 2017 Oct 25. Erratum in: Nature. 2018 May 2;: PMID: 29160308; PMCID: PMC5726555. |
UNIQ535cdb31c9371e74-partinfo-00000005-QINU
