Part:BBa_K3113103
P10SN
This sequence codes for a coiled-coil structure which interacts with the coiled-coil peptide P9SN. We use this construct to load RNA binding proteins into virus-like particles. "Protein coiled-coil (CC) dimers serve as building blocks for modular de novo design of polyhedral protein cages that efficiently self-assemble in vitro and in vivo" (Ljubetic, A., et al. (2017)).
Usage
To increase the modularity of our system we decided to test coiled-coil proteins. These coiled coils mimic the interactions of DNA helix-helix interactions. The coiled-coil protein interaction allows the loading of more than one protein or larger proteins. We are using these helical structures to load RNA binding proteins into exosomes or virus-like-particles.
Biology
"We describe a system analogous to designed DNA nanostructures in which protein coiled-coil (CC) dimers serve as building blocks for modular de novo design of polyhedral protein cages that efficiently self-assemble in vitro and in vivo."[1]
Characterization
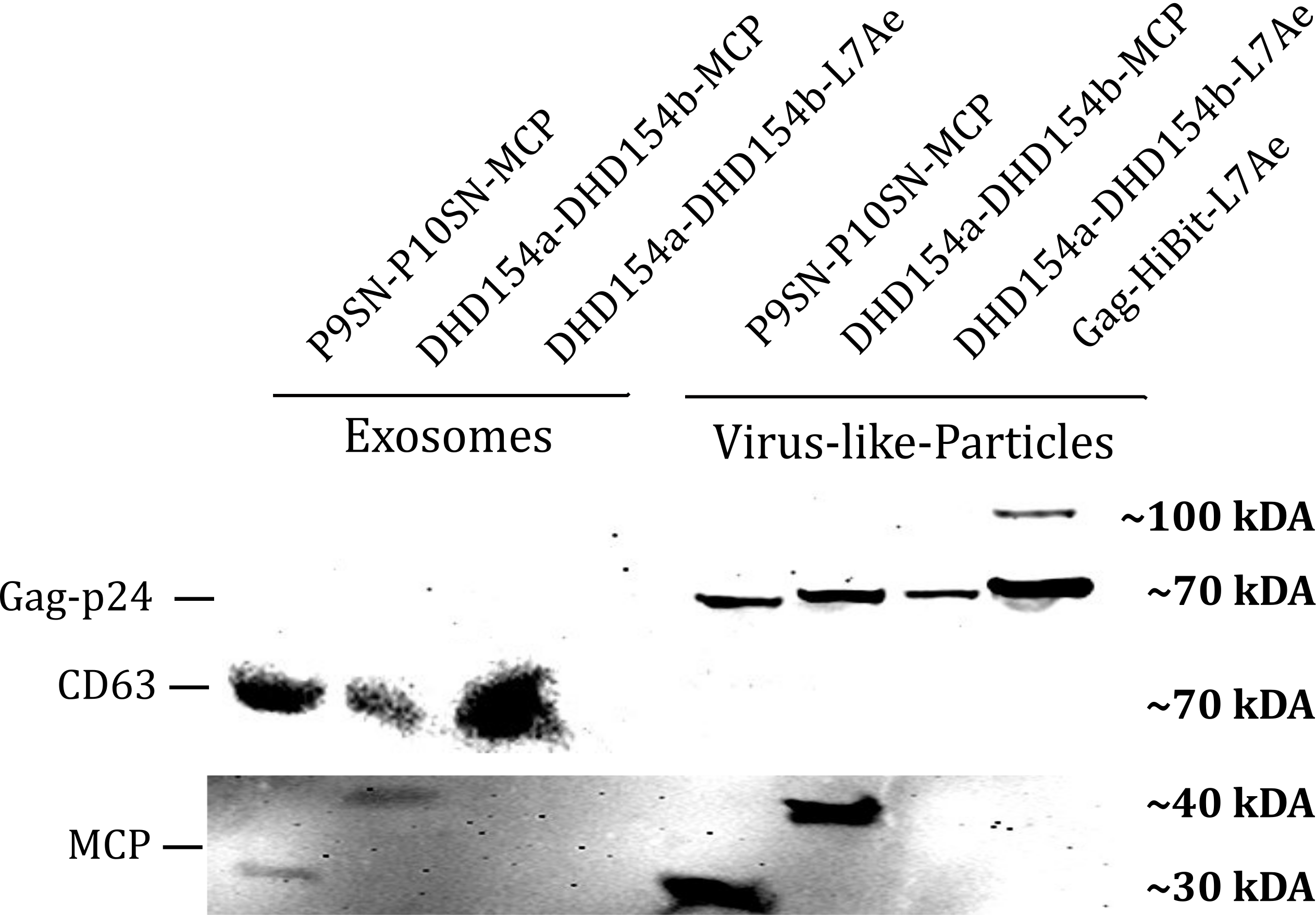
Western Blot
qPCR
Using a coiled-coil system (Figure 7) allowed us to successfully load the two different RNA binding proteins (RBPs) L7Ae or MCP into exosomes to export FLuc mRNA from HEK293T cells. We used the published parallel heterodimer pair P9SN:P10SN (Ljubetič et al. 2017), where P9SN was fused to the C-terminus of the exosome marker protein CD63, and P10SN N-terminal to the RNA binding proteins L7Ae or MCP. The export efficiencies of FLuc mRNA cargo measured by qPCR proved that coiled-coil mediated targeting of the RBPs L7Ae and MCP into exosomes worked. Exosomes formed from CD63-P9SN and loaded with P10SN-L7Ae or -MCP could export about 800 and 400 transcripts per cell, respectively. This also shows that the two RBPs have different export capabilities, with L7Ae exporting twice as many transcripts per cell compared to MCP.

Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
References
- ↑ Ljubetič, A., et al. (2017). "Design of coiled-coil protein-origami cages that self-assemble in vitro and in vivo." Nature Biotechnology 35: 1094.
| None |
