Part:BBa_K338001
Heat Shock Promoter (HSP)
This is a modified BBa_K112400 heat shock promoter in BBa standard.
Usage and Biology
BioBrick Characterization
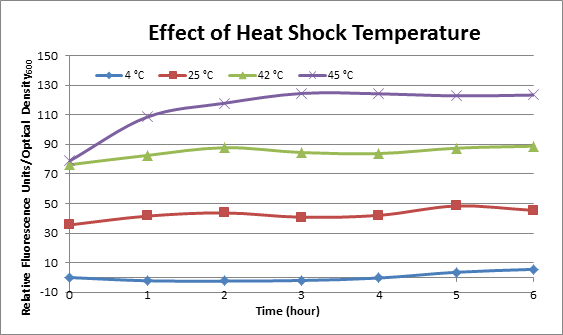
Effect of Heat Shock Temperature
DH5α cells containing HSP-GFP were incubated at 4°C, 25°C, 42°C, and 45°C separately for two hours. Measurements of the fluorescence level of GFP were taken using the plate reader at 20 minute intervals and were averaged over a 1 hour interval. The vertical axis is raw fluorescence units normalized by OD600. Normalized value of 4°C at t=0 was subtracted from all the values to show a relative difference.
This experiment data suggests that heat shock promoter activity increases with heat shock temperature.
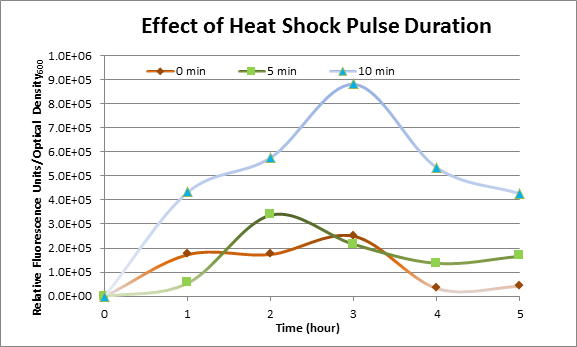
Effect of Heat Shock Duration
DH5α cells containing HSP-GFP were heat shocked at 42°C for 0 min (kept at room temperature), 5 min and 10 min separately. Measurements of the fluorescence level of GFP were taken using the plate reader at 15 minute intervals and were averaged over a 1 hour interval. The vertical axis is raw fluorescence units normalized by OD600.
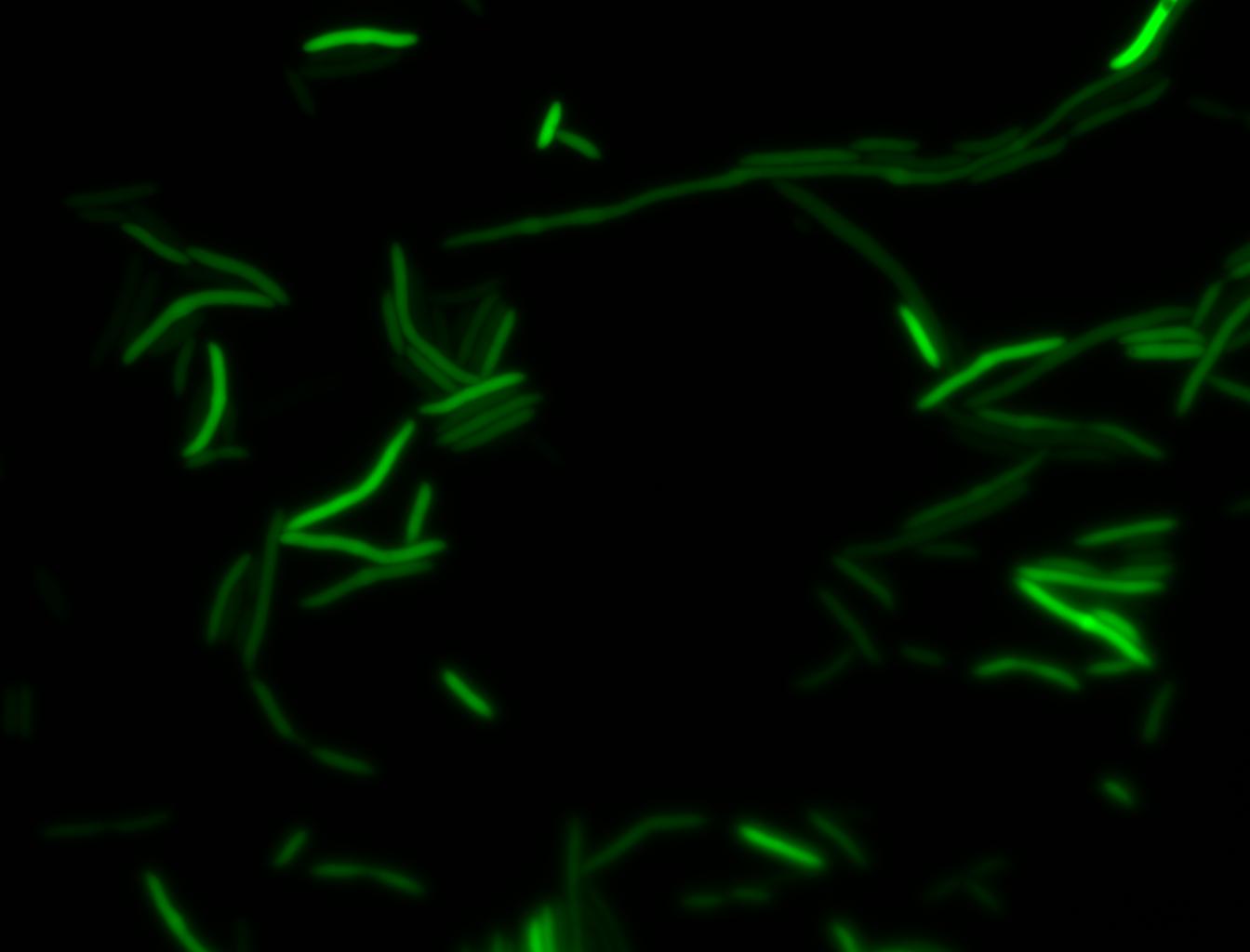
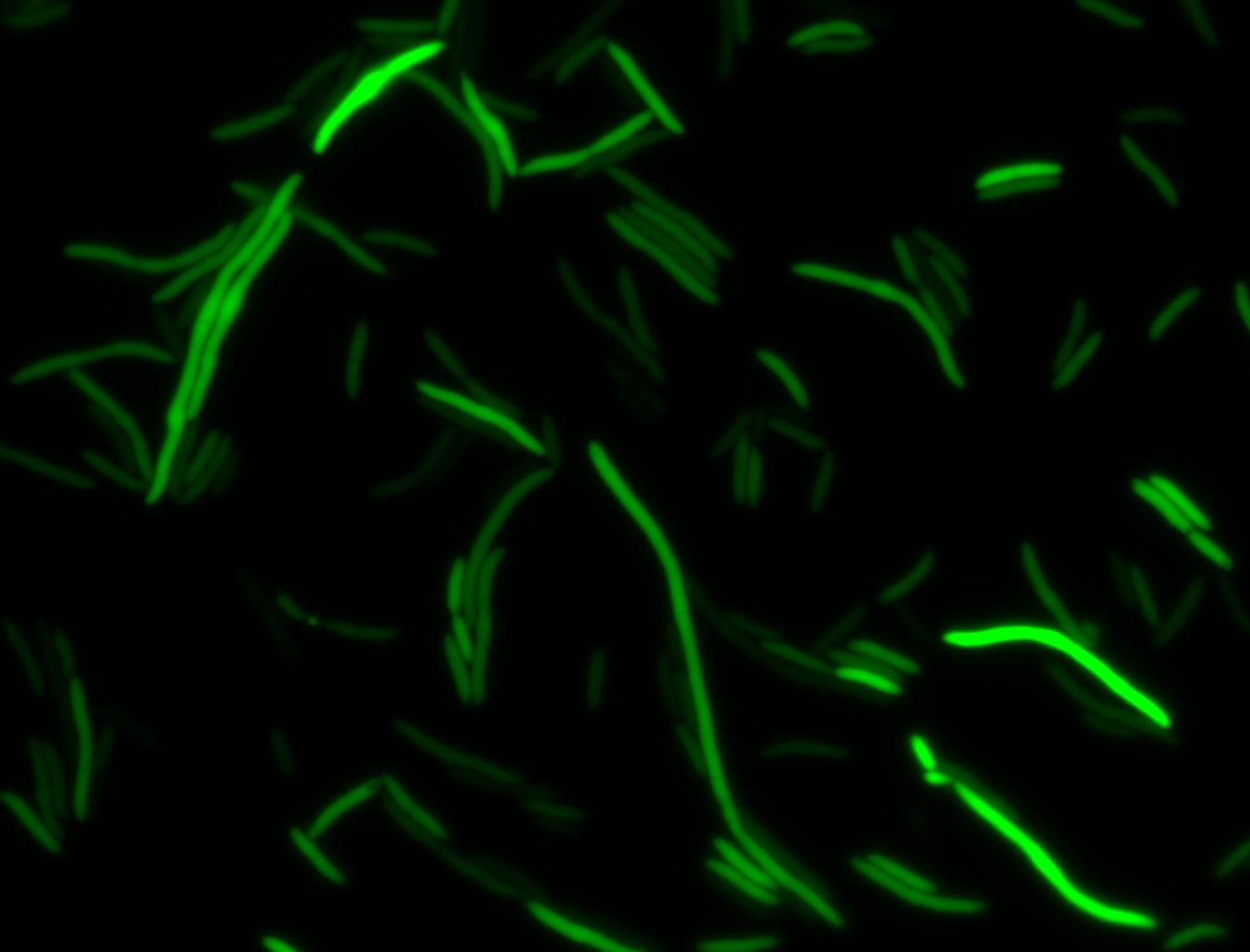
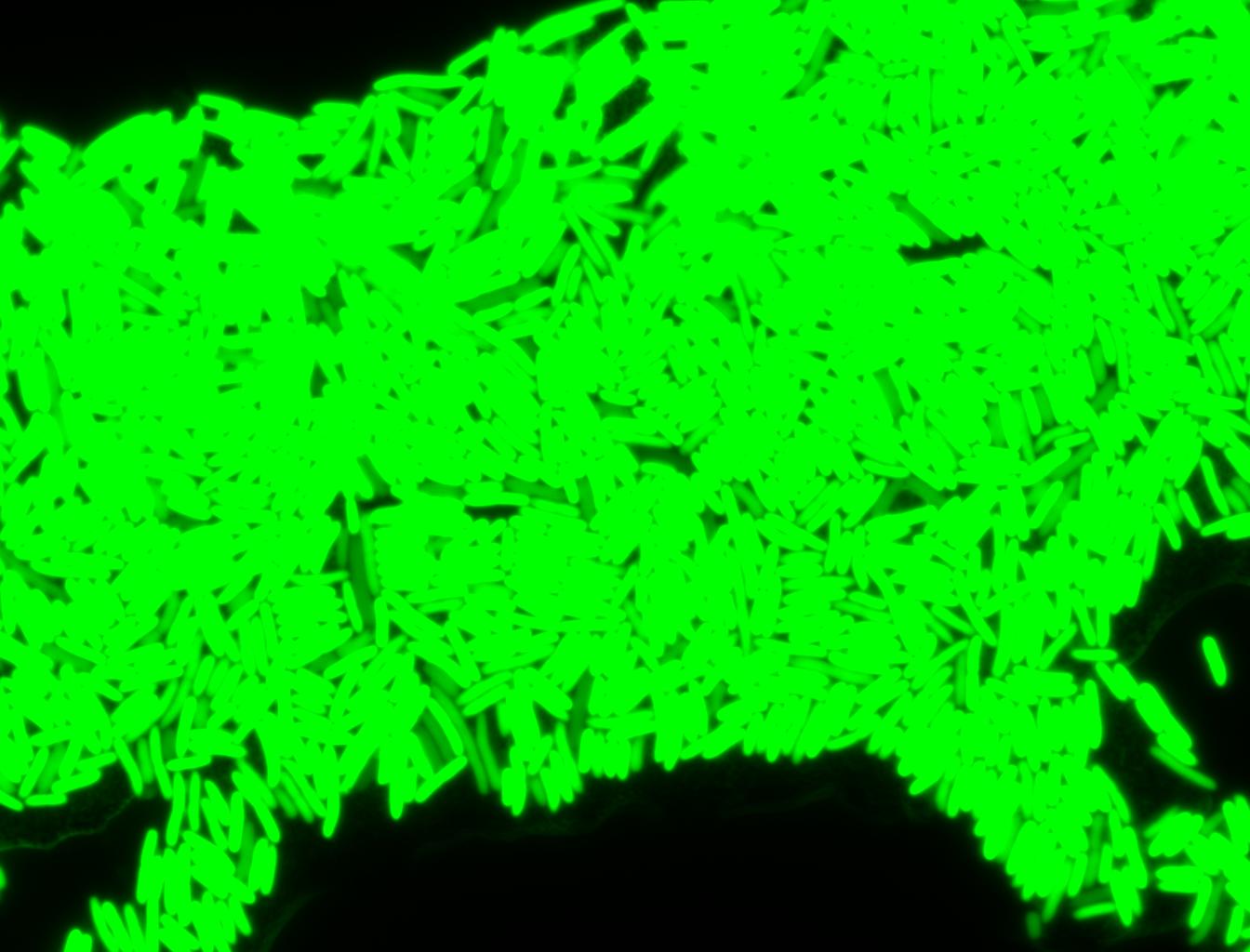
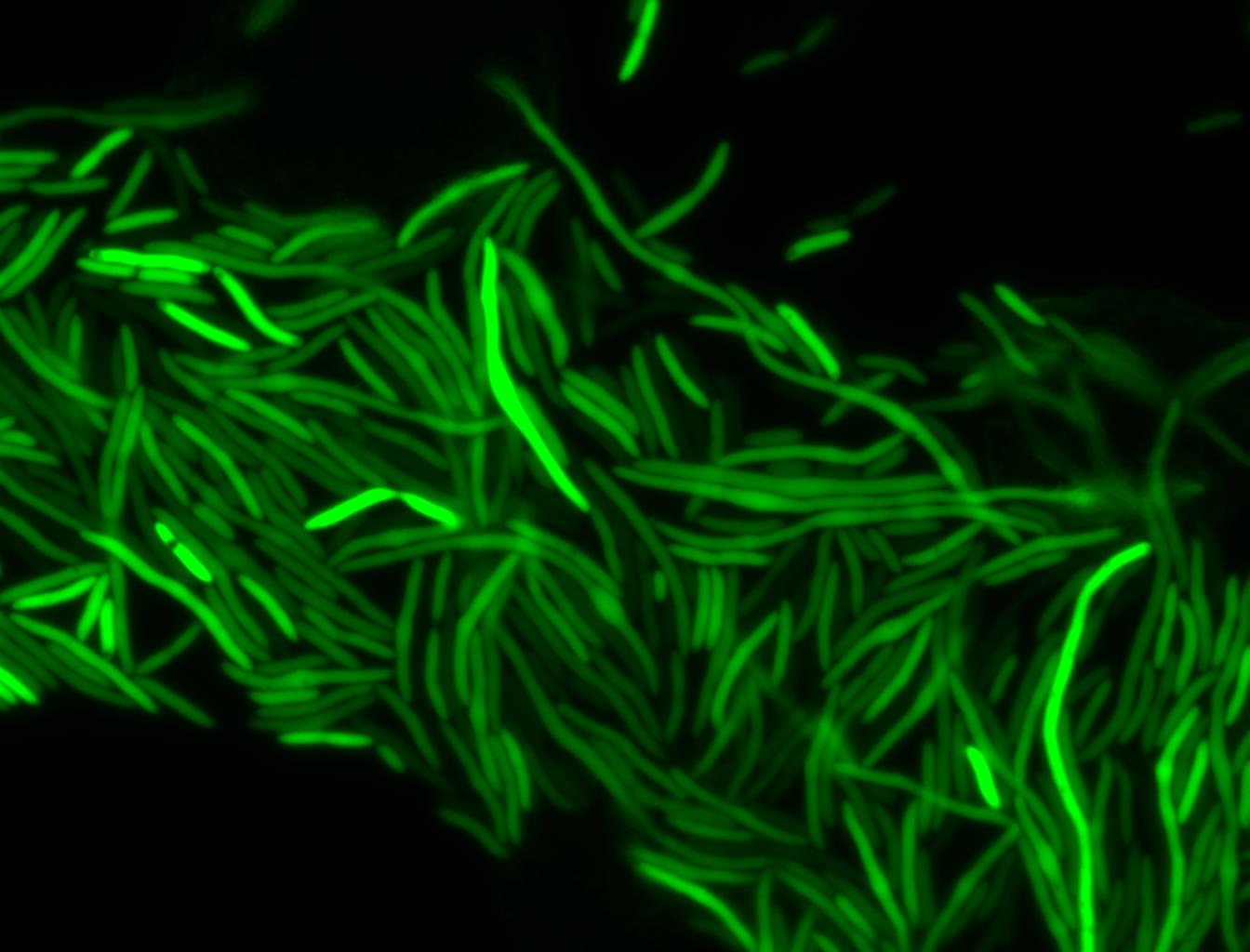
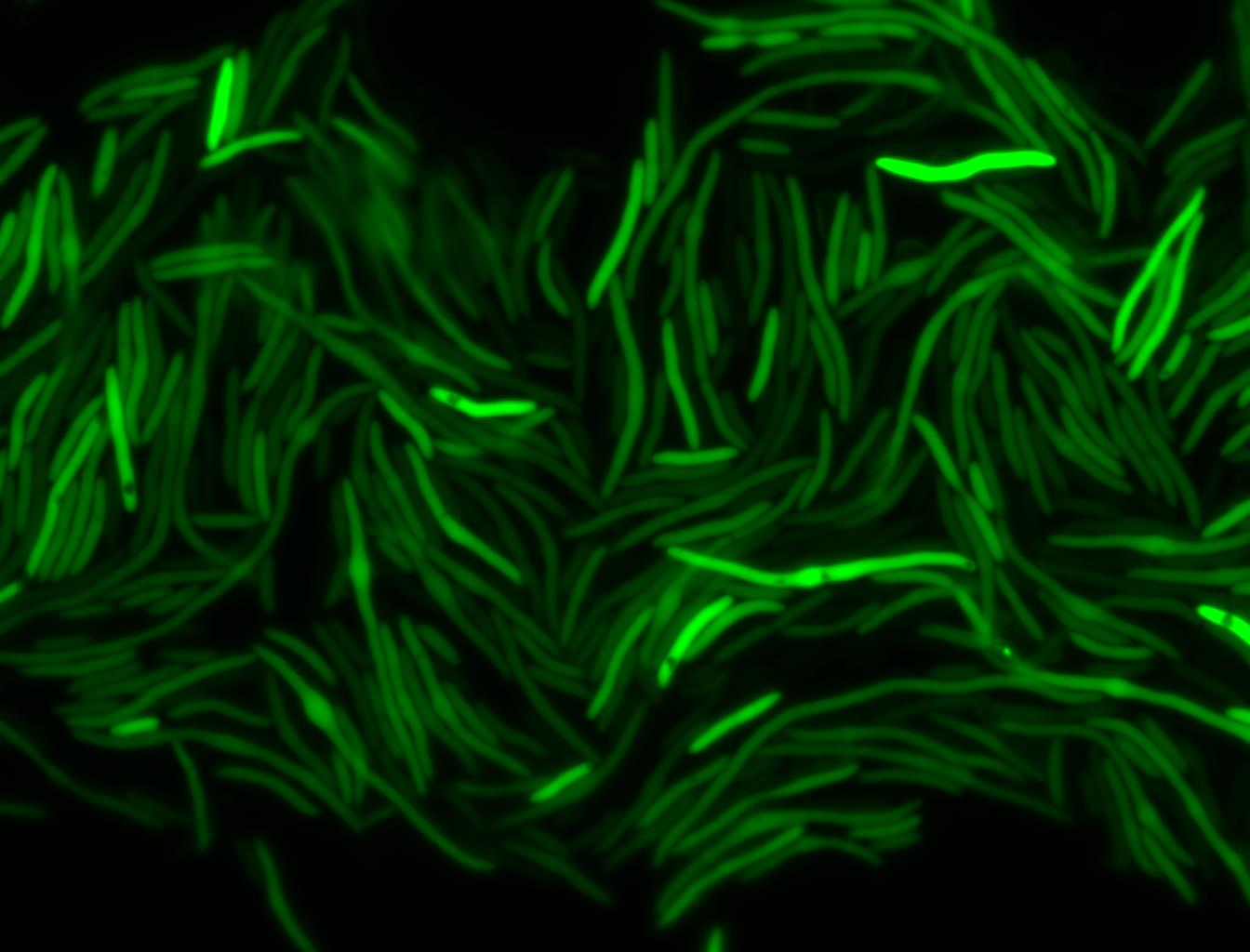
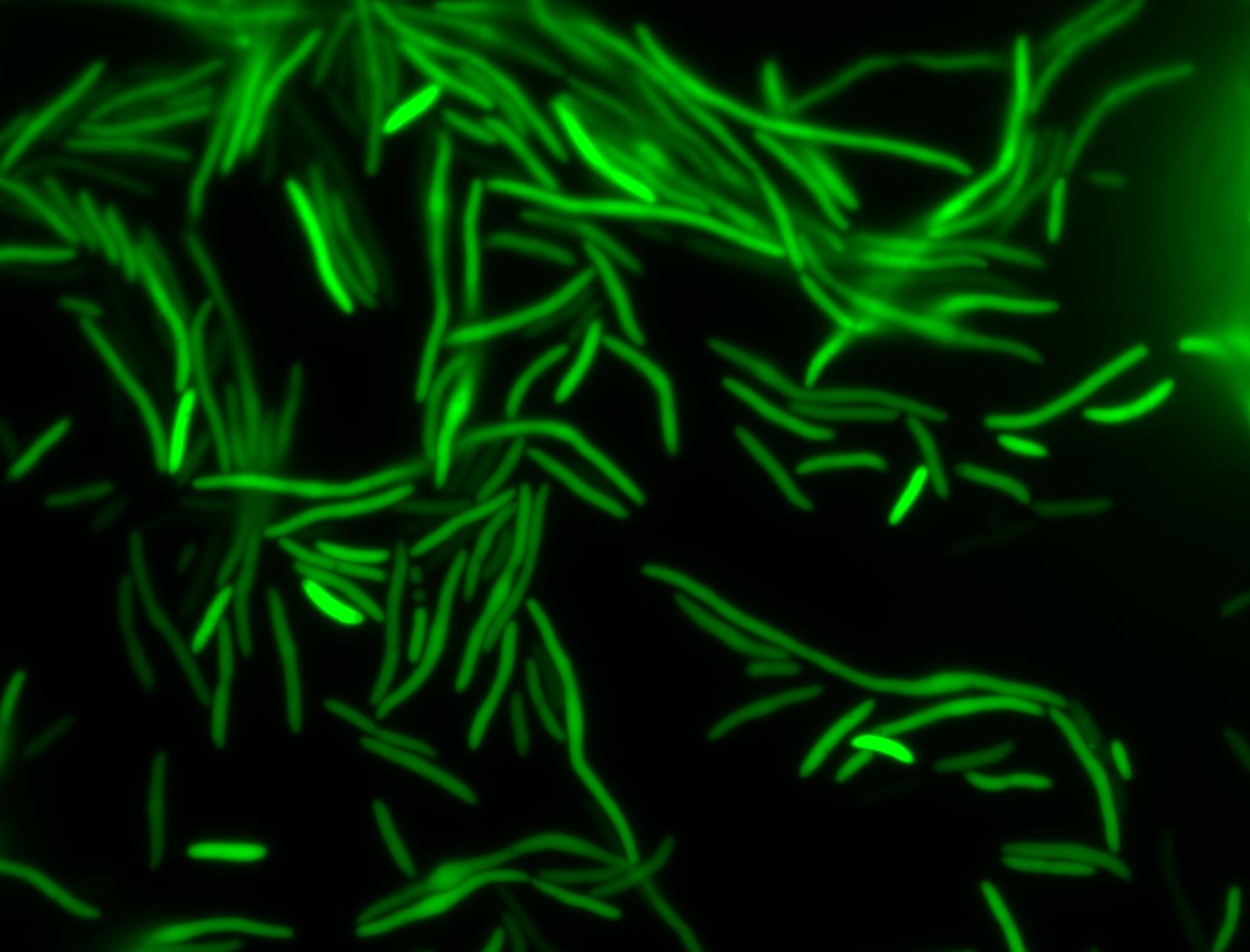
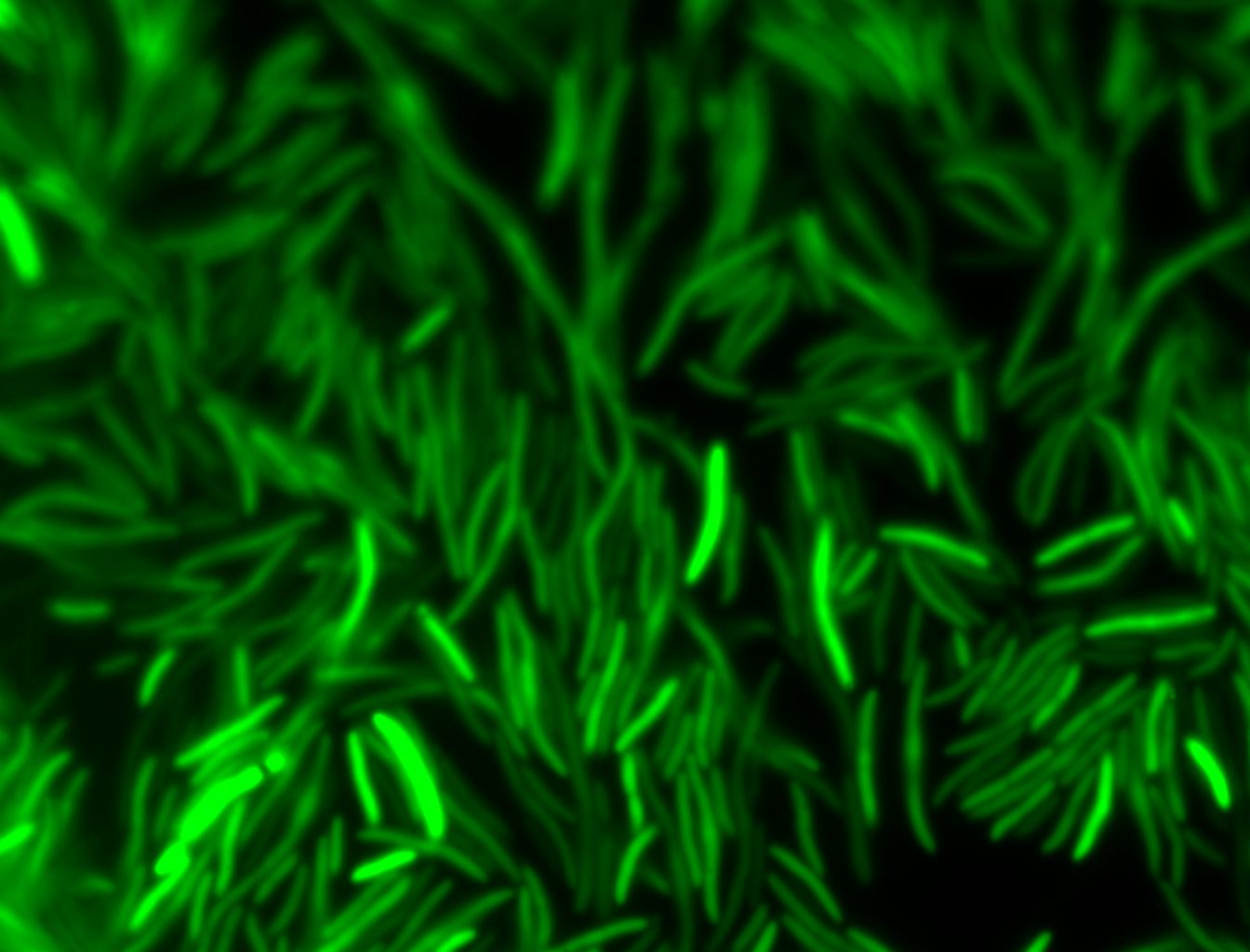
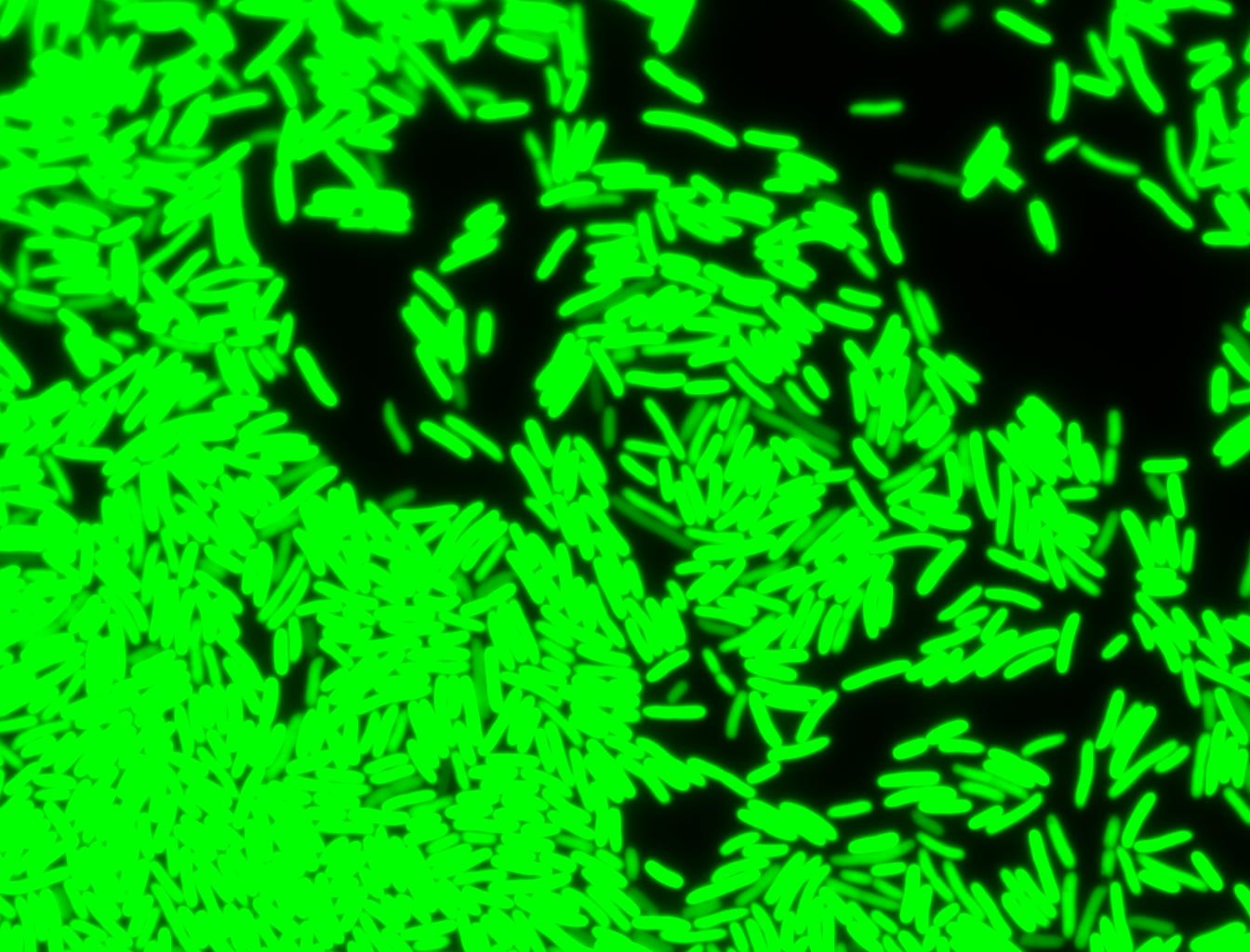
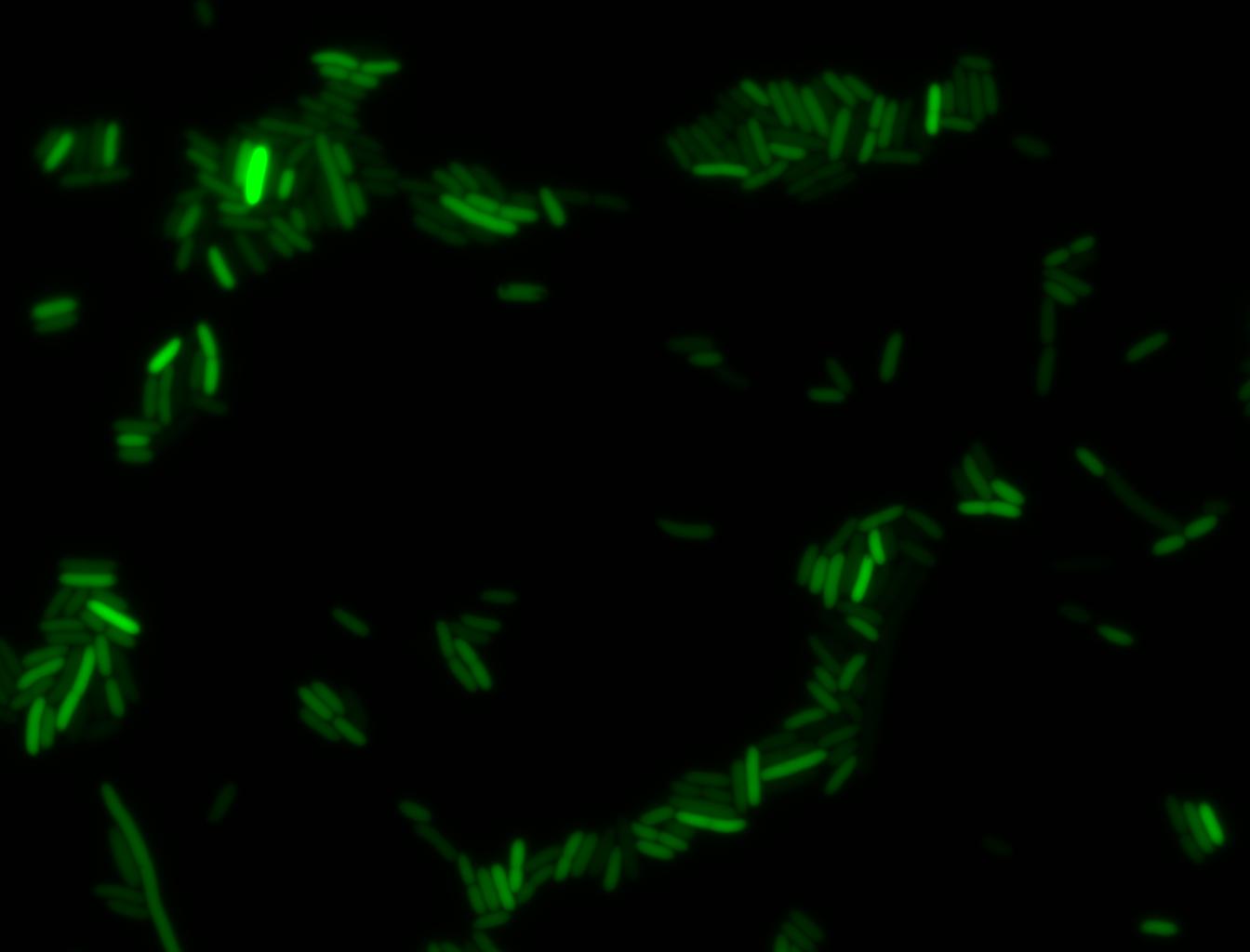
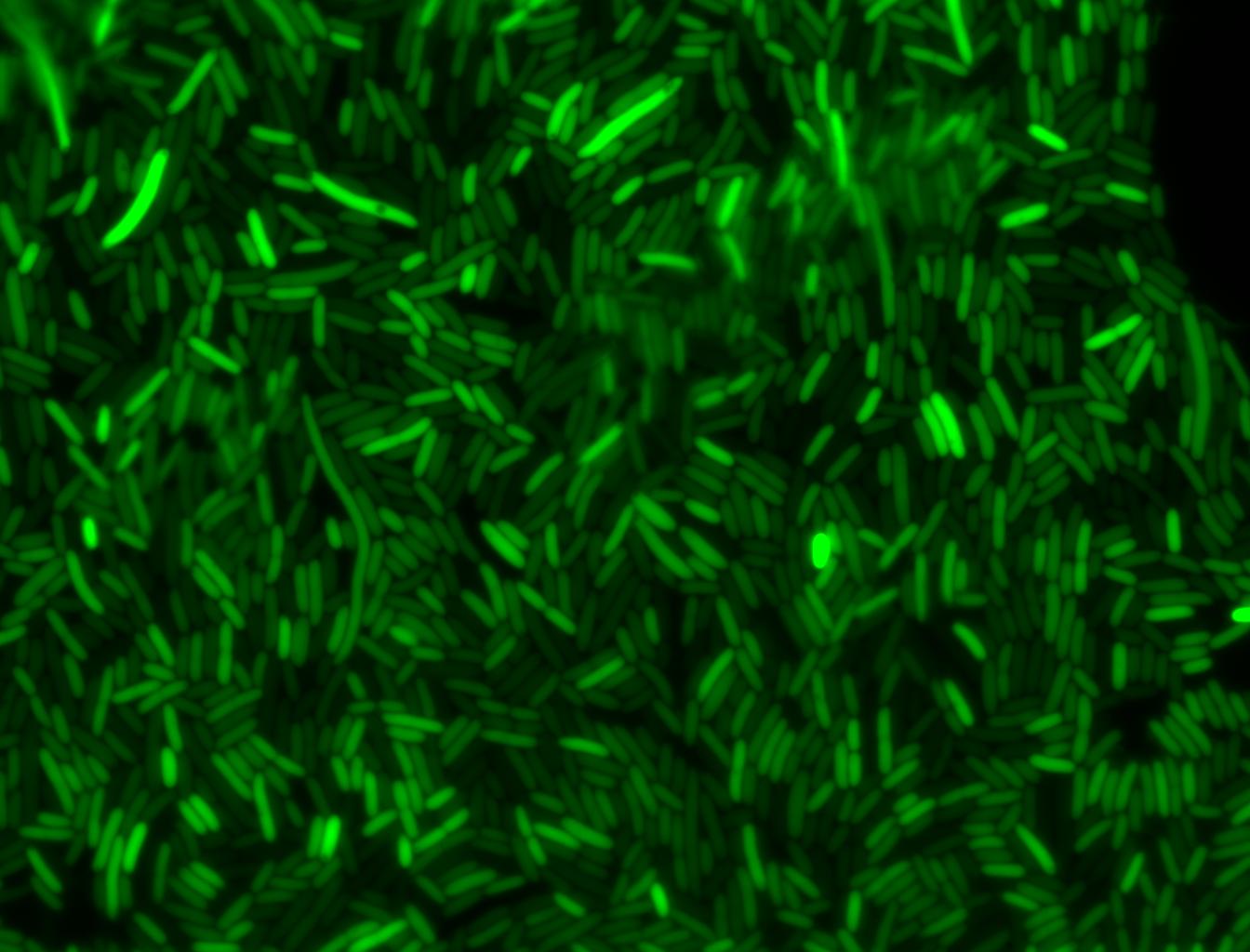
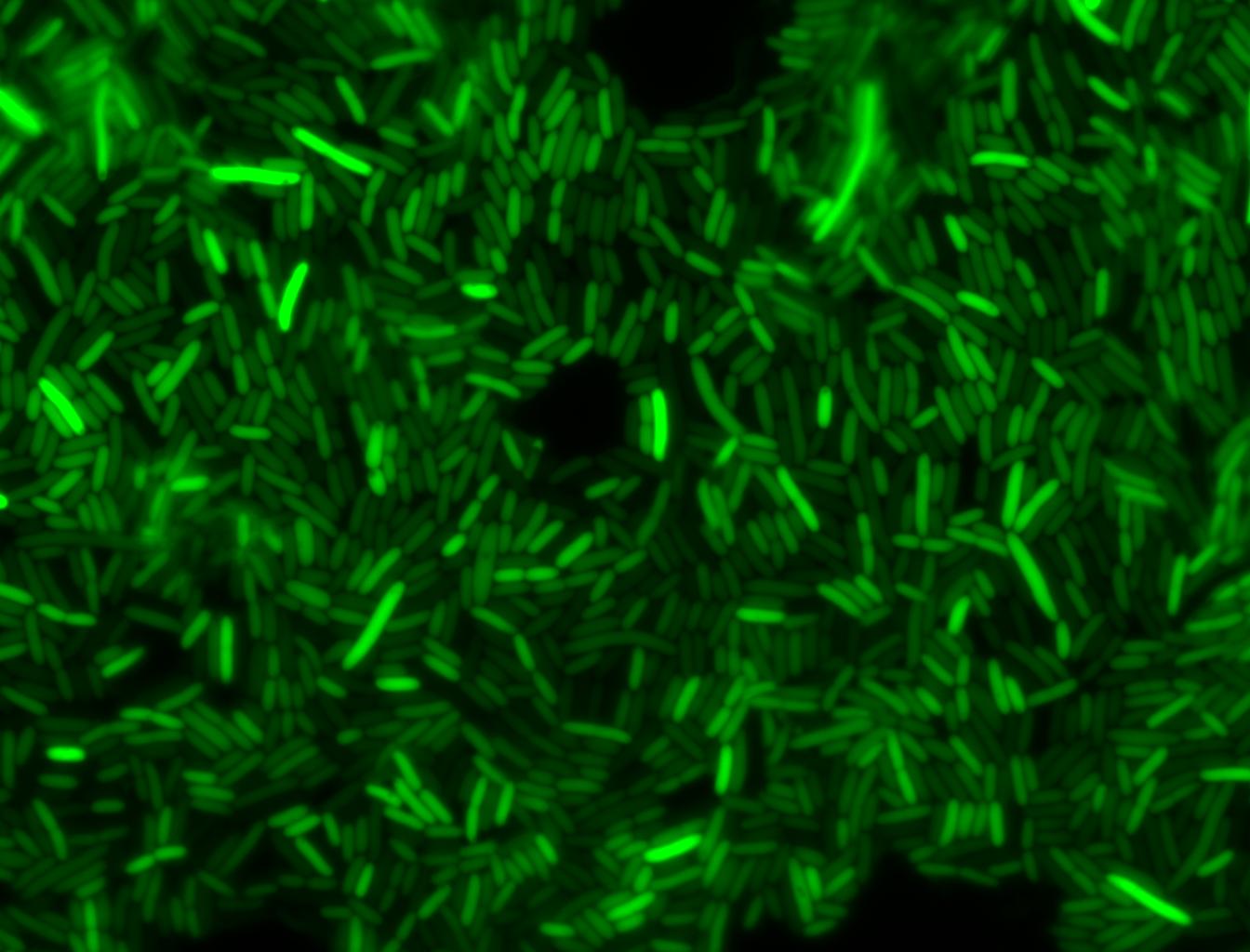
The measurements suggest that a heat shock period of 10 minutes significantly increases the production of GFP, while a heat shock period of 5 minutes leads to a similar GFP production level as the untreated cells. Furthermore, the production of GFP shows a decreasing trend after 3 hours suggesting that the heat shock promoter was only activated for a certain time period. DH5α cells contained in the images below-visualized by an optical microscope with a GFP-specific filter- were incubated at 42°C for 10 minutes.
Images of Cells at t=0 min (Before Heat Shock)
Control: cells containing only GFP at t=0
Images of Cells at t=10 min
Control: cells containing only GFP at t=10
Images of Cells at t=60 min
Control: cells containing only GFP at t=60
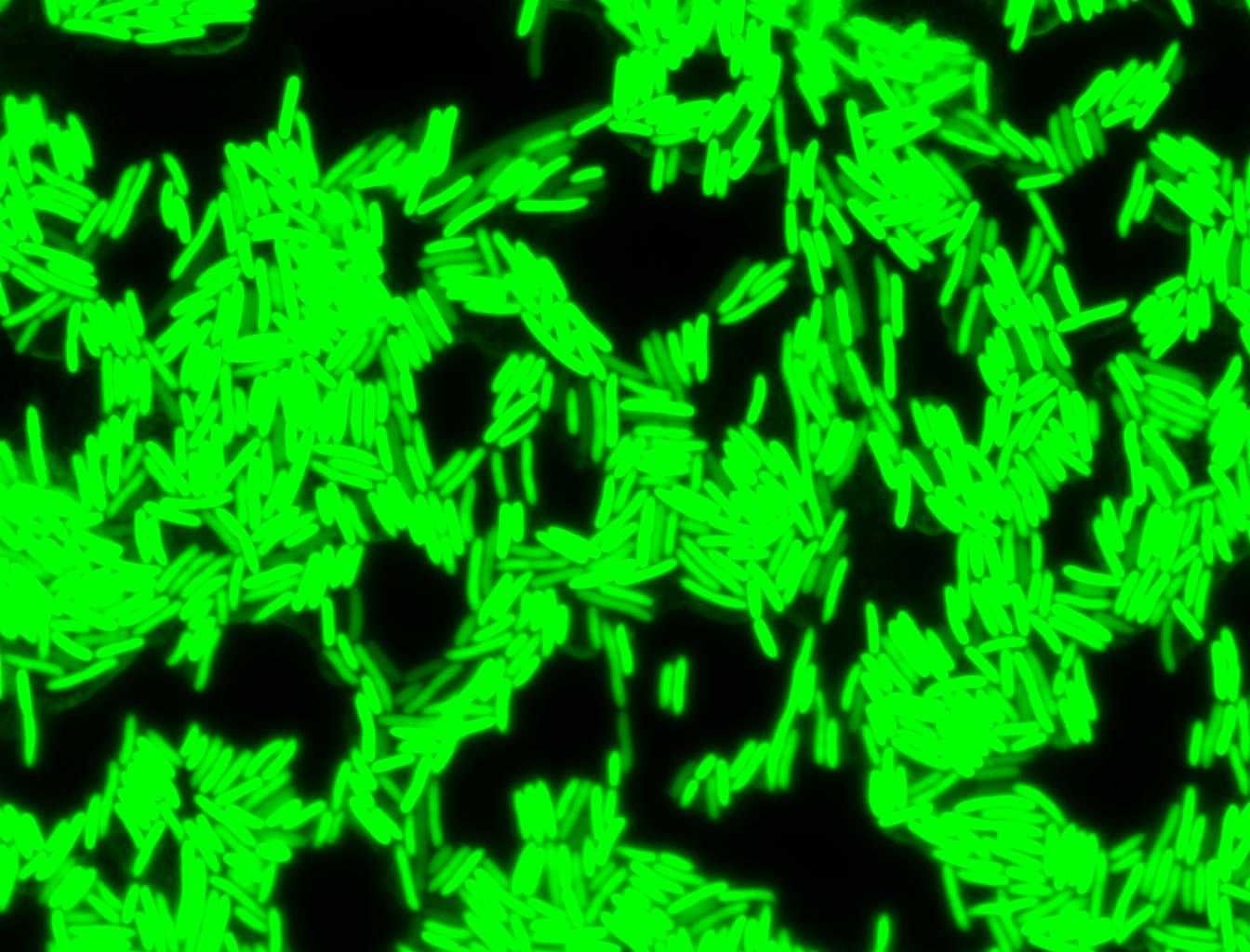
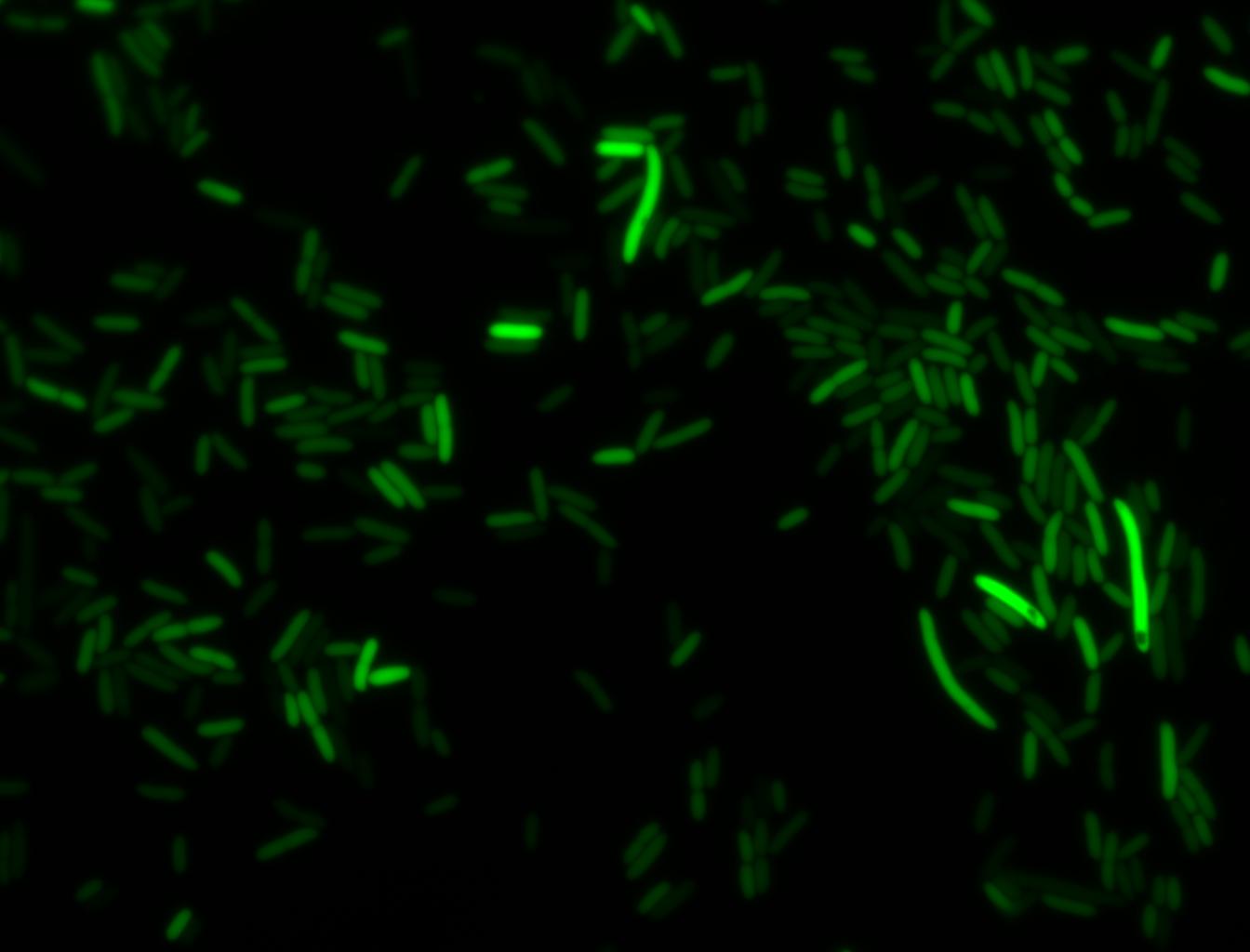
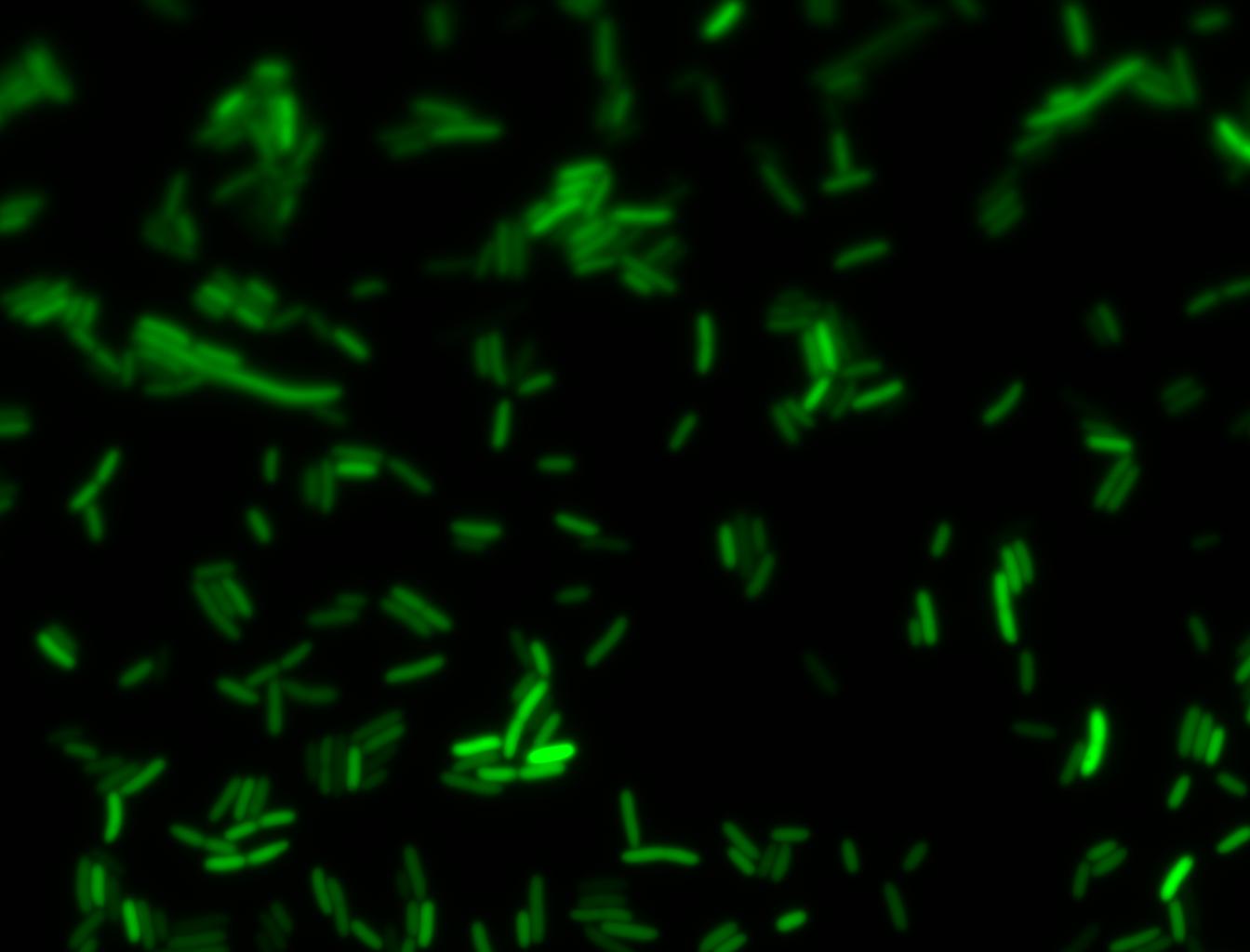
Glucose
An interesting observation was made: when adding 20 mM of glucose to the media at the time of growth,the cells are no longer morbidly elongated at the time of imaging.
Images of Cells at t=0 min (Before Heat Shock)
Images of Cells at t=10 min
Images of Cells at t=60 min
NOTE: All images were captured at 100x magnification.
Contribution
Group: Valencia_UPV iGEM 2018
Author: Adrián Requena Gutiérrez, Carolina Ropero
Summary: We adapted the part to be able to assemble transcriptional units with the Golden Gate assembly method
Documentation:
In order to create our complete [http://2018.igem.org/Team:Valencia_UPV/Part_Collection part collection] of parts compatible with the Golden Gate assembly method, we made the part BBa_K2656001 which is this part adapted to the Golden Gate technology.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
//direction/forward
//chassis/prokaryote/ecoli
//promoter
//regulation/positive
| negative_regulators | |
| positive_regulators |