Part:BBa_K1761002
Outer Membrane Protein X (OmpX) with BsoBI-linker and NanoLuc
OmpX is an outer membrane protein with the C- and N-termini in the intracellulair domain. To be able to use OmpX as a scaffold, a unnatural amino acid needs to be introduced. This can be done by implementing the amber stop codon TAG in one of the loops of OmpX via a mutation. With a specific tRNA an azide-functionalized amino acid can be built in, which can be used for the SPAAC click chemistry reaction with DBCO functionalized groups. The BsoBI linker is a 213 bp long flexible GGSGGS linker. Using the restriction enzyme BsoBI, the linker can become 45 bp shorter. NanoLuc is a small sized and bright luciferase. Under the addition of furimazine, the NanoLuc luciferase generates a bioluminescece emmission spectrum with a maximum around 460 nm.
Usage and Biology
OmpX, or Outer Membrane Protein X, belongs to a family of highly conserved bacterial proteins that promote bacterial adhesion to and entry into mammalian cells. It presents both C- and N-termini in the intracellular domain (see Figure 1). OmpX consists of an eight-stranded antiparallel all-next-neighbor β barrel. The core of the protein consists of an extended hydrogen-bonding network of highly conserved residues (see Figure 1). [1]
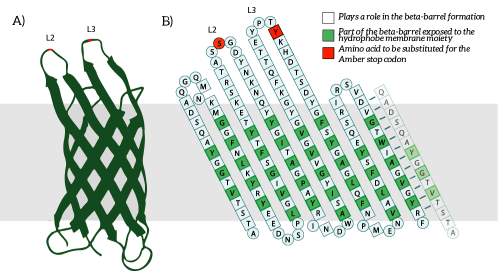
Figure 1: A) The OmpX protein structure has been elucidated through NMR and X-ray crystallography. B) The square residues are important for the secondary structure of OmpX. To keep the structure intact, we introduce an amber stop codon in one of the protruding loops. Figure 5B is adapted from [1].
NanoLuc is a small sized and bright luciferase protein engineered for optimal performance as a bioluminescent reporter. The small size and bright luminescence bring exquisite sensitivity to reporter assays and other bioluminescence applications. The luciferase naturally occurs in the organism Oplophorus gracilirostis.
Gene Design
This construct consists of three parts, namely OmpX, a BsoBI-linker, and NanoLuc. OmpX is already described as a single part, and that same part, with the amber stop codon TAG incorporated for the integration of the unnatural amino acid, is used for this construct. For more information, see part BBa_K1761000 [1].
Between OmpX and mNeonGreen we inserted a BsoBI-linker. This is a 213 bp long flexible GGSGGS-linker with two BsoBI-restriction sites. These sites give the possibility to shorten the linker with 45 bp. The BsoBI-linker is inspired by the article "Quantitative Understanding of the Energy Transfer between Fluorescent Proteins Connected via Flexible Peptide Liners" by T.H. Evers et al from 29 August 2006. [2]
These three parts together give a construct with OmpX (1) as transmembrane protein and NanoLuc (2) as intracellular domain (see Figure 2).
Figure 2: Schematical overview of the expressed OmpX with NanoLuc in the outer membrane of E.coli.
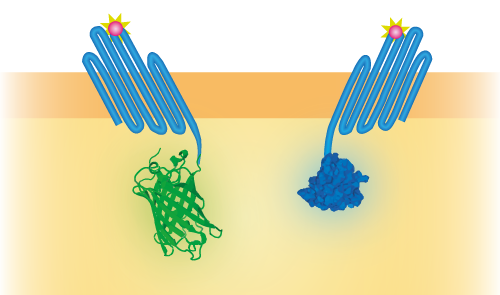
For all our experiments we used pETDuet-1 as a vector. This vector has two multiple cloning sites, namely MCS-1 and MCS-2. To be able to use this part as a sensor, we inserted both OmpX – NanoLuc and OmpX – mNeonGreen into this vector, both in different multiple cloning sites. In this way, NanoLuc and mNeonGreen can function as a BRET pair (see Figure 3).
Figure 3: Schematical overview of the expressed OmpX with mNeonGreen and OmpX with NanoLuc in the outer membrane of E.coli. The mNeonGreen and NanoLuc domains form a BRET pair.
Sequence
The sequence of our OmpX - NanoLuc part (see Figure 4) has been verified by sequencing at StarSeq. It contains the prefix and suffix with the correct restriction sites (EcoRI, XbaI, SpeI and PstI). OmpX is 513 bp long, the BsoBI-Linker is 213 bp long and NanoLuc 513 bp.
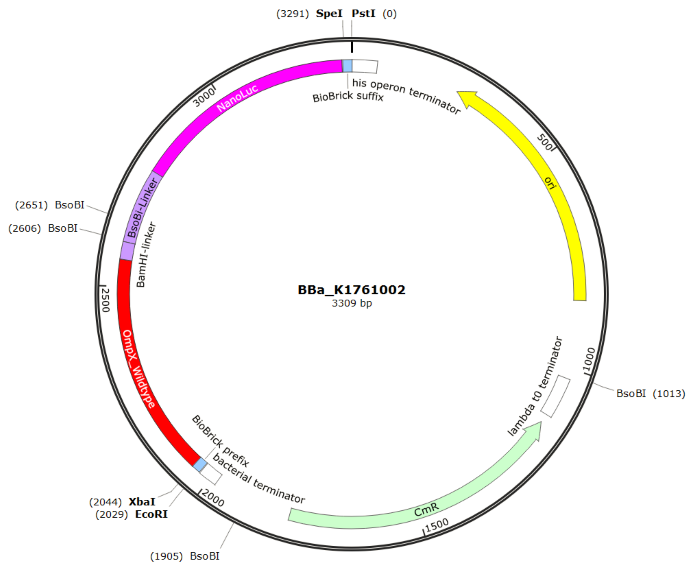
Figure 4: Snapgene plasmid overview of the BioBrick part BBa_K1761002. It shows the pSB1C3 vector with the prefix (containing the restriction sites EcoRI and XbaI), wildtype OmpX, the BsoBI linker, NanoLuc and the suffix (containting the restiction sites SpeI and PstI).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 769
- 1000COMPATIBLE WITH RFC[1000]
Characterization
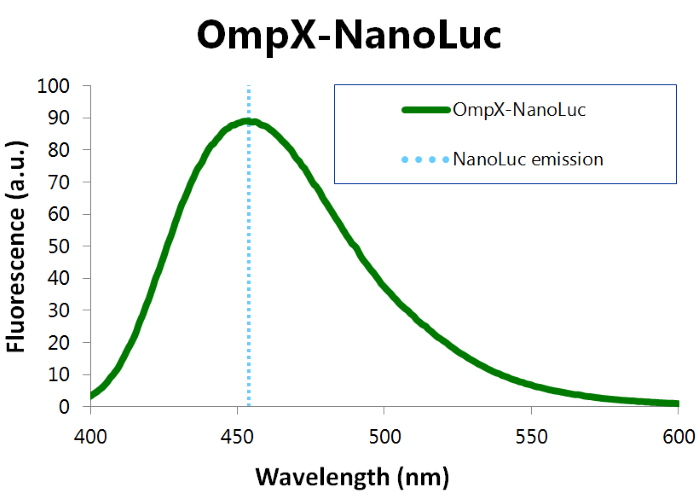
When mutated with the amber stop codon TAG, OmpX can covalently bind anything by using a bio-orthogonal “click” reaction. The presence of NanoLuc in this construct can be tested by adding furimazine and read out its emission spectrum. If it is present, there should be a maximum around 460 nm.
For all the experiments we used the following vectors: pETDuet-1 with a construct inserted (OmpX + intracellular protein) and pEVOL-pAzF (tRNA + tRNA synthetase). Both vectors were transformed into BL21(DE3). The expression was introduced by adding arabinose, IPTG and the unnatural amino acid.
DBCO-PEG4-TAMRA Confirmation
To confirm whether OmpX is in the membrane and whether or not the unnatural amino acid is being incorporated into OmpX, DBCO-PEG4-TAMRA was used. TAMRA is a fluorescent dye that can be used to verify the “click” reaction. If the unnatural amino acid is present, DBCO-PEG4-TAMRA should “click” to the transmembrane protein OmpX and stay there. This can be analyzed with FACS. For more information about how to perform FACS experiments, see our Protocol Page [http://2015.igem.org/Team:TU_Eindhoven/Project/Protocols].
OmpX – NanoLuc gave the following results after clicking with DBCO-PEG4-TAMRA (see Figure 5). From this it can be concluded that OmpX is in the membrane and that the “click” reaction works.
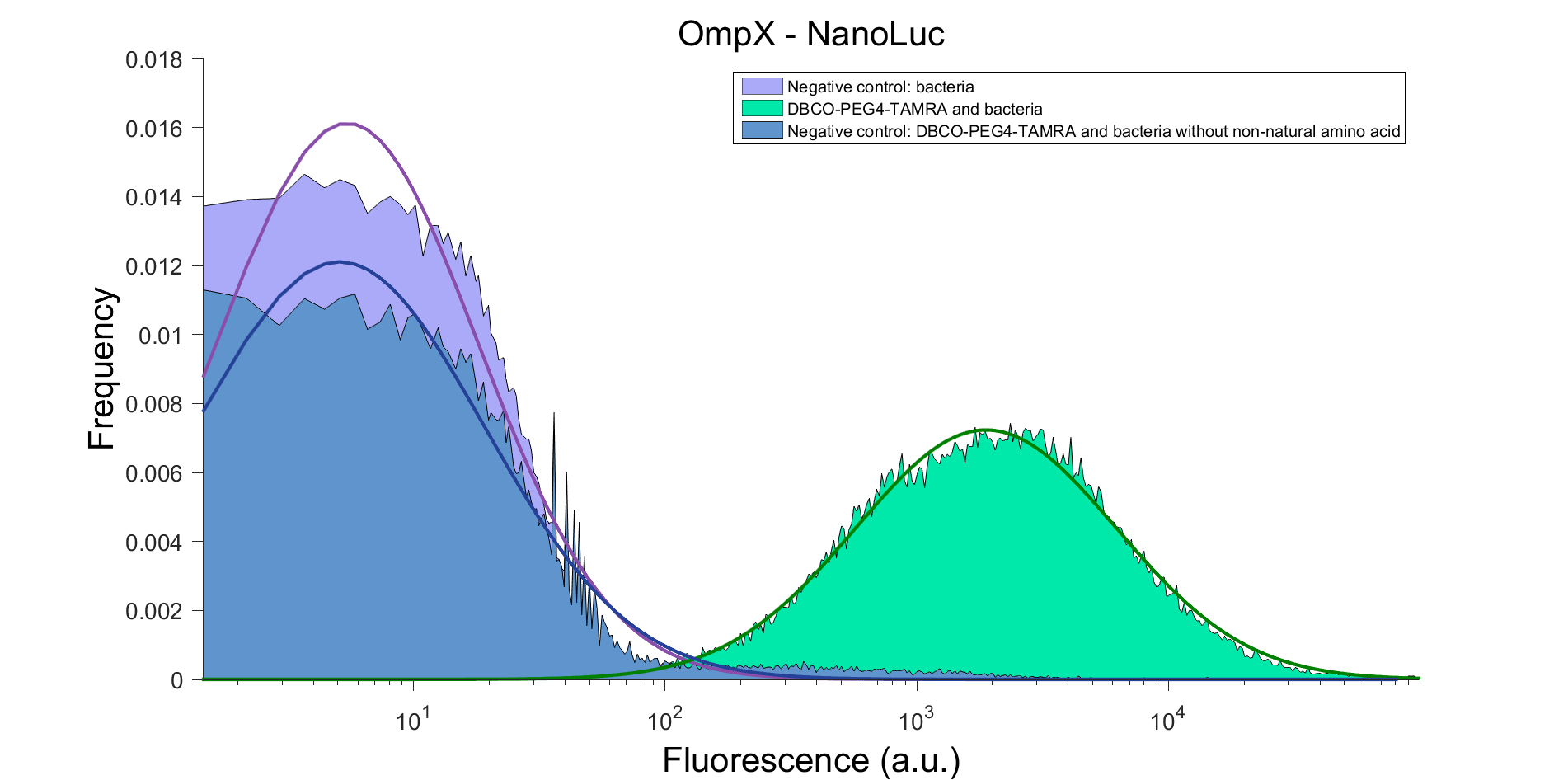
Figure 5: FACS result of OmnP - NanoLuc
Bioluminescence Confirmation
To confirm whether NanoLuc is present in the bacteria, a bioluminescence measurement was performed (see Figure 6). For more information about how to perform a bioluminescence measurement, see our Protocol Page [http://2015.igem.org/Team:TU_Eindhoven/Project/Protocols].
Figure 6: Bioluminiscence results NanoLuc
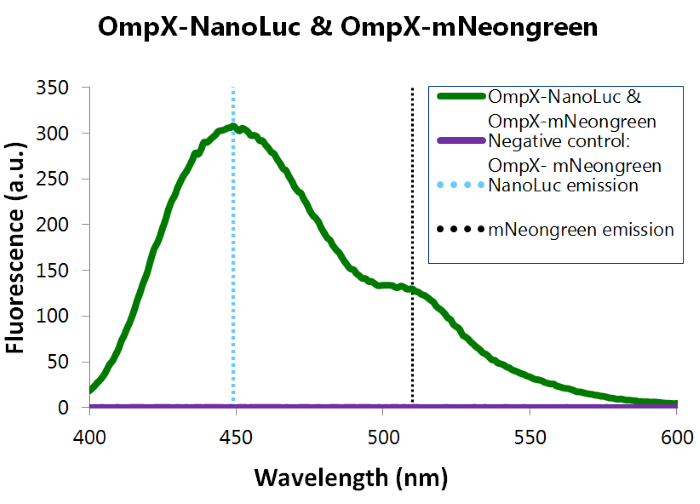
OmpX-NanoLuc's & OmpX-mNeonGreen's presence in the cells were tested by adding Nano-Glo substrate to the cells and measuring bioluminescence with the spectrophotometer. Figure 7 shows that OmpX-NanoLuc shows a peak characteristic for NanoLuc, indicating NanoLuc's presence within the cells. Moreover, the spectrogram shows a distinct shoulder near 517 nm, the emission wavelength of mNeongreen. Since no laser was used, excitation of mNeongreen can only be accomplished by NanoLuc so BRET (Bioluminescence resonance energy transfer) occured. This signal is measured when no click reaction is performed on the complex, meaning that this can be seen as the background noise of the sensor of iGEM team TU Eindhoven 2015.
Figure 7: Bioluminiscence results NanoLuc and mNeonGreen.
References
[1] J. Vogt and G. E. Schulz, “The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence.,” Structure, vol. 7, no. 10, pp. 1301–9, Oct. 1999.
[2] T.H. Evers et al, “Quantitative Understanding of the Energy Transfer between Fluorescent Proteins Connected via Flexible Peptide Linkers.,” Biochemistry, vol. 45, no. 44, pp. 13183-92, Nov. 2006.
//function/reporter/light
| emission | 460 nm |