Part:BBa_K1355001:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K1355001
Experiments and Results as a BIOSENSOR (BBa_K1355001 + BBa_E0840) = BBa_K1355002
The experiment to quantify GFP expression induced by Hg was made according to the protocol “Quantification of Green Fluorescent Protein (GFP) induced by different concentrations of mercury in Escherichia coli DH5α”. DH5α transformed with BBa_K1355002 was inoculated in LM (LB with low concentration of NaCl) liquid medium with chloramphenicol and grew until the Optical Density was 0.4 to 0.6abs (measured on spectrophotometer at 600 nm wavelength). After cell growth, an aliquot of 500μl in 5 eppendorf tubes (2ml) was taken and then added mercury chloride in order to achieve the concentrations of: 0.01 µg/ml, 0.02 µg/ml, 0.1 µg/ml, 0.2 µg/ml, and 1 µg/ml. The samples were incubated at 37°C on shaker. We collected each eppendorf tube at time 1 (01:30 hours of incubation), time 2 (03:00 hours of incubation) and time 3 (04:30 hours of incubation). Every sample was centrifuged at 12000g for 3 minutes and the pellet washed with TN Buffer (Nacl 0.15M + Tris HCl 10mM) and then re-suspended with 500μl of the same buffer. The same process was made to the bacterium without construction as a control to GFP expression/intensity. GFP expression was measured using the Hidex Chameleon spectrofluorimeter with excitation filter 340 nm and emission filter 500 nm wavelength. The Optical Density was measured simultaneously. All samples were analyzed in triplicate.
The graph represented on Figure 1 shows the Optical Density of transformed DH5α with BBa_K1355002 in different Hg concentrations in function of time:
Figure 1. Optical Density measured in the four given times, at mercury chloride concentrations of 0 µg/ml, 0.01 µg/ml, 0.02 µg/ml, 0.1 µg/ml, 0.2 µg/ml, and 1 µg/ml.
In these condition cell growth increases along time. The highest values correspond to bacteria not exposed to mercury or to small concentrations, as in 0 µg/ml, 0.01 µg/ml and 0.02 µg/ml. Suggesting a harmless condition to bacteria. However, cell growth decreases at higher concentrations as, 0.1 µg/ml, 0.2 µg/ml and especially 1µg/ml, giving to bacteria a hard time for development.
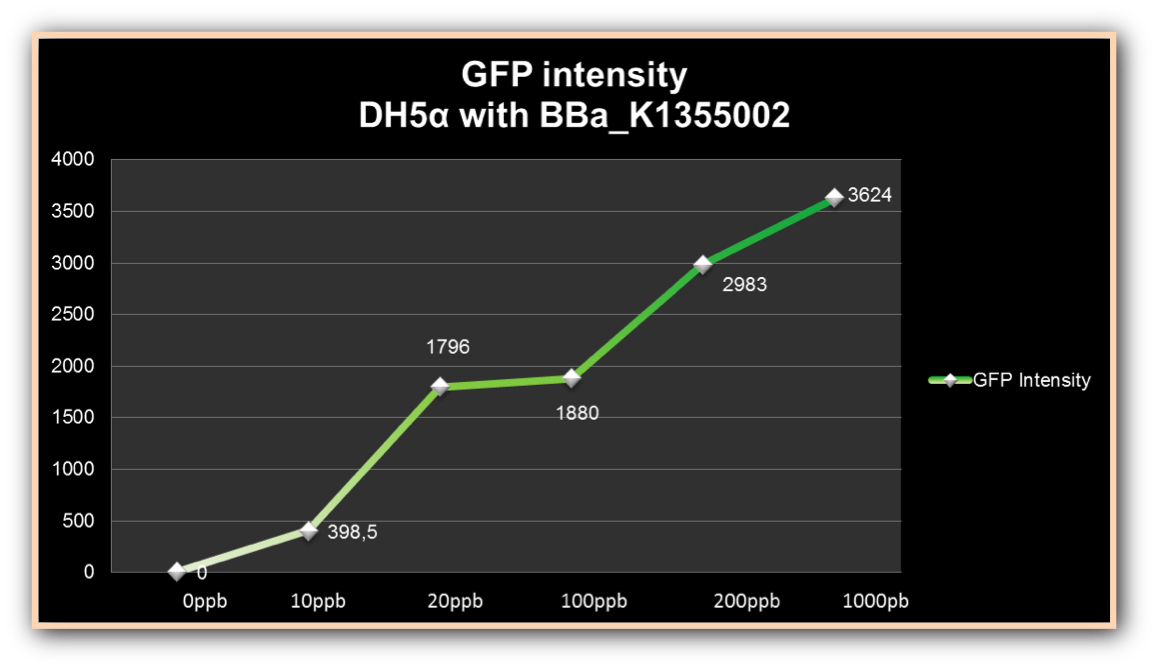
The graph represented on Figure 2 shows the fluorescence emitted by DH5-alpha induced by different Hg concentrations in function of the time; and the graph represented on Figure 3, shows the ratio between fluorescence emitted and Optical Density.
Figure 2. GFP fluorescence intensity in the four given times, at mercury chloride concentrations of 0 µg/ml, 0.01 µg/ml, 0.02 µg/ml, 0.1 µg/ml, 0.2 µg/ml, and 1 µg/ml.
Figure 3. GFP fluorescence per cell growth ratio in the four given times, at mercury chloride concentrations of 0 µg/ml, 0.01 µg/ml, 0.02 µg/ml, 0.1 µg/ml, 0.2 µg/ml, and 1 µg/ml.
Fluorescence can be observed in bacteria exposed to small concentrations, as 0.01µg/ml and 0.02µg/ml, but has low intensity. Fluorescence levels presents medium intensity in the higher concentration as consequence of cell death. Fluorescence intensity increased more than 480% in 0.2µg/ml concentrations, compared to fluorescence intensity from 1µg/ml, even in cell growth reduction, demonstrating its efficiency to induce mer promoter.
Experiments and Results as a BIOACCUMULATOR (BBa_K1355001 + BBa_K346004) = BBa_K1355003
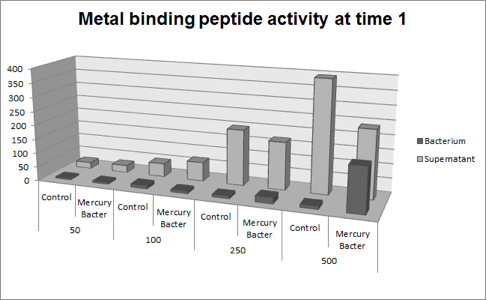
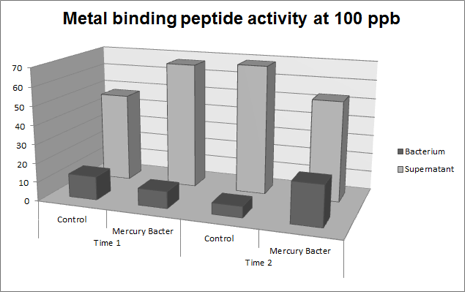
The experiment for Hg bioaccumulation was made according to the protocol “Quantification of Mercury bio accumulated by metal binding peptide (MBP) in recombinant DH5-alpha in different Hg concentrations”. DH5-alpha transformed with BBa_K1355003 was inoculated in LM (LB with low concentration of NaCl) liquid medium with chloramphenicol and grew until the optical density was 0.4 to 0.6abs (measured on spectrophotometer at 600 nm wavelength). After cell growth, an aliquot of 400μl was taken and distributed in 4 eppendorf tubes (1.5ml) and then added mercury chloride in order to achieve the concentrations: 50 ppb, 100 ppb, 250 ppb and 500 ppb. The samples were incubated at 37°C on shaker. We collected each eppendorf tube at time 1 (01:30 hours of incubation) and time 2 (03:00 hours of incubation). After the designated time, both were centrifuged at 12000g for 3 minutes and the supernatant recovered (LM medium). We washed the pellet with TN Buffer (Nacl 0.15M + Tris HCl 10mM) and then re-suspended with 400μl of the same buffer. To measure bio accumulated Hg, we need to quantify the Hg inside and outside of bacterium after the incubation/exposure time. So we collected and measured the amount of Hg in LM medium supernatant recovered and bacterium re-suspended in TN Buffer. For this we used the equipment Direct Mercury Analyzer (DMA-80). As a control to normal Hg bio accumulated in bacteria, we used DH5-alpha transformed with BBa_K1355002 (Hg bio detector device) which does not present the metal binding peptide. We also measured the Optical Density of each sample. The graph represented on Figure 1 shows the amount of Hg in supernatant (LM medium recovered) and in bacterium (DH5-alpha transformed with BBa_K1355003) at the time 1 (01:30 hours of incubation);
Figure 1: Metal binding peptide activity after 01:30 hours of incubation in five given concentrations of mercury chloride: 50 ppb, 100 ppb, 250 ppb e 500 ppb
It can be observed that the amount of Hg in the Mercury Bacter bioaccumulator increases according to the raise of Hg concentration. The amount of Hg increased 22 times comparing the 50 ppb sample with 500 ppb sample. In the 500 ppb Hg concentration the control bacterium just accumulated 2 per cent of total Hg amount. On the contrary, Mercury Bacter accumulated 40 per cent of total Hg amount in just 01:30 hours of incubation!!! The data keeps raising on the time 2! Check it out!
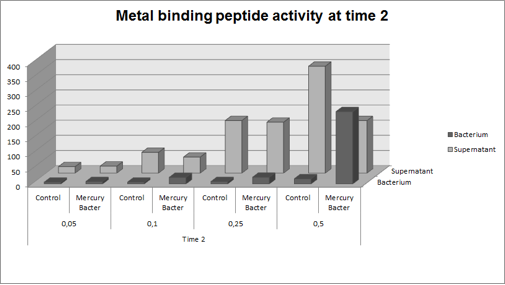
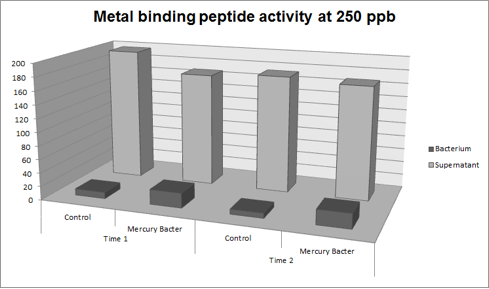
The graph represented on Figure 2 shows the amount of Hg in supernatant (LM medium recovered) and in bacterium (DH5-alpha transformed with BBa_K1355003) at the time 2 (03:00 hours of incubation);
Figure 2: Metal binding peptide activity after 03:00 hours of incubation in five given concentrations of mercury chloride: 50 ppb, 100 ppb, 250 ppb e 500 ppb.
It can be observed that the amount of Hg in Mercury Bacter bioaccumulator, increases according to the time of incubation. The amount of Hg increased 30 times comparing the 50 ppb sample with 500 ppb sample. In the 500 ppb Hg concentration the control bacterium just accumulated 4% of total Hg amount. Instead, Mercury Bacter accumulated 58% of total Hg amount in just 03:00 hours of incubation!!! The data can be analyzed individually in each concentrations samples as shown in graphs represented in the figures below:
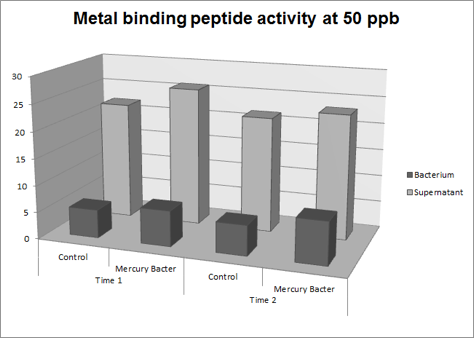
Figure 3: Metal binding peptide activity at 50 ppb in time 1 and 2;
Figure 4: Metal binding peptide activity at 100 ppb in time 1 and 2;
Figure 5: Metal binding peptide activity at 250 ppb in time 1 and 2;
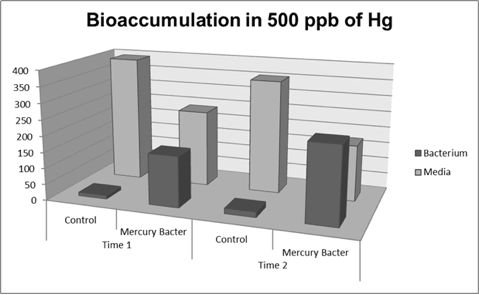
Figure 6: Metal binding peptide activity at 500 ppb in time 1 and 2
Experiments and Results as a BIOREMEDIATOR (BBa_K1355001 + BBa_K1355000) = BBa_K1355004
The experiment for Hg bioremediation was made according to the protocol “Cell growth quantification of mercury resistant Escherichia coli DH5α at differents Hg concentrations by Optical Density”. DH5-alpha transformed with BBa_K1355004 was inoculated in LM (LB with low concentration of NaCl) liquid medium with 0.05μg/ml of mercury chloride and 34μg/ml of chloramphenicol. The inoculum was incubated in shaker overnight at 37°C. After cell growth, we measured Optical Density (O.D.) of the cell culture in spectrophotometer at 600 nm wavelength. A standard quantity aliquot of bacterial suspension was taken in six falcons (50ml) with 10 ml of LM liquid medium and then added mercury chloride in order to achieve the concentrations: 0μg/ml, 1 μg/ml; 2.5 μg/ml; 5 μg/ml; 10μg/ml; 20 μg/ml. The samples were incubated at 37°C on shaker. We collected each sample at the given times, 1 (02:30 hours of incubation), time 2 (04:30 hours of incubation), time 3 (06:30 hours of incubation), time 4 (17 hours of incubation), time 5 (24 hours of incubation) to measure O.D. on spectrophotometer. As control, we used DH5-alpha transformed with BBa_K1355002 (Hg bio detector device) which does not present coding regions for the mercuric ion reductase. We also measured Optical Density of each sample. After cell growth measurement, the samples were frozen to subsequently quantification of Hg remediated by the Mercury Bacter.
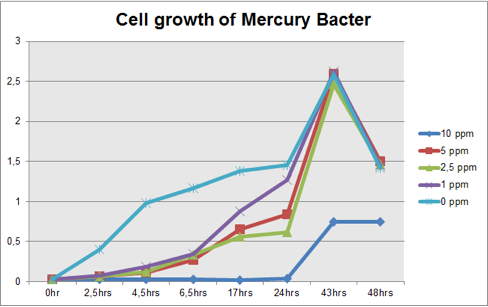
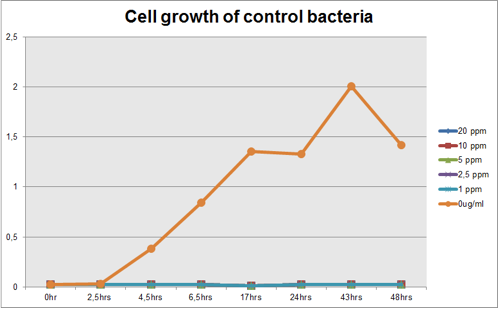
The graph represented on Figure 1 and Figure 2 shows Cell growth of Mercury Bacter and of the control bacteria in different Hg concentrations in function of time, respectively;
Figure 1: Cell growth of Mercury Bacter in different Hg concentrations in function of time;
Figure 2: Cell growth of control bacteria in different Hg concentrations in function of time
Comparing the graphs shown on figure 1 and 2, it can be observed that the biobrick BBa_K135504 provided resistance to high Hg concentrations. Control bacteria did not present resistance to Hg concentration equal or higher to 1 ppm. On the contrary, Mercury Bacter grew up even in 10 ppm Hg concentrations. Interestingly, at the concentration of 10 ppm Mercury Bacter needed 24 hours to exhibit increase in cell growth, and after 48 hours it maintained the same OD, unlike the others. At 1 ppm, 2.5 ppm and 5 ppm Mercury Bacter performed an overwhelming cellular growth after 24 hours of exposure to Hg, reaching approximately the same cellular growth as the non-exposed bacteria!!
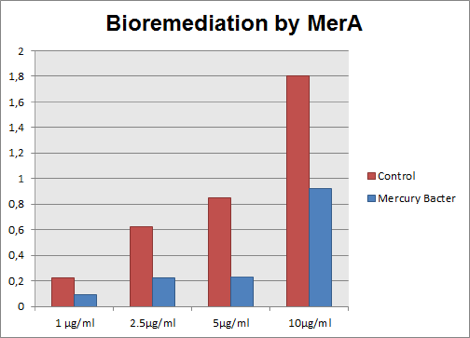
The graph represented on Figure 3 compares Hg concentration in LM medium after 48 hours of experiment, between medium with bacteria used as control and engineered Mercury Bacter.
Figure 3: Hg concentration in LM medium after 48 hours of cell growth in control bacteria and in the genetically engineered Mercury Bacter.
In the graph above it can be observed that our super Mercury Bacter bioremediated! In action, Mercury Bacter reduced 72% of the mercury levels, in comparison to control at the sample 5 ppm! In 1 ppm, Even though on 10 ppm concentration, where bacteria had a slow growth rate only after 24 hours, it bioremediated 48.02%! These results demonstrate our constructions’ efficiency, both for biobrick BBa_K1355001 as a promoter regulated by MerR protein and to BBa_K1355000 as MerA encoding gene!
User Reviews
UNIQ5c21a8bb84b1379d-partinfo-00000000-QINU UNIQ5c21a8bb84b1379d-partinfo-00000001-QINU