Part:BBa_K1412716
GFP generator with J23101
This part is consist of Anderson promoter J23101 and GFP generator BBa_E0240. It can be used for characterization of promoter J23101. When in backbone vector pSB3K3, the copies is low, so we need observe it carefully. While in backbone vector pSB1C3, the expression is strong, so we can observe it easily.
Usage and Biology
When we want to measure the expression intensity of a promoter, the most concise method is connect it with a GFP generator. Then we just need an device to measure the fluorescent, and compare their fluorescent intensity with each other.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12INCOMPATIBLE WITH RFC[12]Illegal NheI site found at 7
Illegal NheI site found at 30 - 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI.rc site found at 706
Experimental data
Verfication
Figure 1. Enzyme digestion verification of devices BBa_J23115 + BBa_E0240 and BBa_I20260
1. 1kb Marker;
2. Double restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device BBa_J23115 + BBa_E0240 (constructed by [http://2014.igem.org/Team:XMU-China# XMU-China]).
3. Mono-restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device BBa_J23115 + BBa_E0240 (constructed by [http://2014.igem.org/Team:XMU-China# XMU-China]).
4. Double restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device BBa_I20260 (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]).
5. Mono-restriction enzyme digestion ([http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]) with device BBa_I20260 (reconstructed by [http://2014.igem.org/Team:XMU-China# XMU-China]).
Results and discussion:
For device BBa_I20260, it’s very abnormal that we get puzzling results with double enzymes digestion by [http://en.wikipedia.org/wiki/Restriction_enzyme Xba I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I]. The target segment seems vanished. However, if we use [http://en.wikipedia.org/wiki/Restriction_enzyme EcoR I] and [http://en.wikipedia.org/wiki/Restriction_enzyme Pst I] instead, we find that the segments, whose backbone is PSB3k3, generated by mono-restriction digestion is about 1000 bp longer than that generated by double restriction enzyme digestion, and the devices have been verified by DNA sequencing. We can also get segments slightly shorter than 1000 bp which generated by double digestion, and those segments are highlighted by red frames. So we confirm that device BBa_I20260 is correct. We took our actual measurement with the reconstructed device.
Figure 2. GFP of different device
1: K1412999 in DH5α;
2: K1412716 in DH5α;
3: K1412716 (connect by us) in DH5α;
4: K1412924 in DH5α.
We cultured the bacteria in the LB medium 12 hrs at 37℃ with shaking at 200 rpm in the table concentrator, then we transferred 1 ml solution to a 1.5 ml centrifuge tube, 10000 rcf, centrifuge 1 min. We did this again, finally we get the bacteria precipitate. From the figure, we can found, at the visible light, the K1412924 emit light green color, while the K1412716 (connect by us) and K1412716 emit a light yellow color, and K1412999 emit a color close to white.
Figure 3. GFP of different device under the UV-light
1: K1412924 in DH5α;
2: K1412716 (connect by us) in DH5α;
3: K1412716 in DH5α;
4: K1412999 in DH5α.
We cultured the bacteria in the LB medium 12 hrs at 37℃ with shaking at 200 rpm in the table concentrator, then we transferred 1 ml solution to a 1.5 ml centrifuge tube, 10000 rcf, centrifuge 1 min. We did this again, finally we get the bacteria precipitate. Under the UV-light, we can observe clearly, the K1412924 can emit bright green fluorescence, while the K1412716 (connect by us) and K1412716 have a light green fluorescence, and the green fluorescence from K1412999 is so weak that we can’t observe.
Measurement
Figure 4. The plot of optical density versus time
From the plot of optical density versus time, we can conclude that the growth rate of bacteria is lower with time. We measured the sample three times parallelly, and we can know that the reproducibility of the data is acceptable. When we compare with BBa_K1412924 and BBa_K1412999, we can get that their growth rate are almost equal.
Figure 5. The plot of RFUs versus time
From the plot of RFUs versus time, we can conclude that RFUs grow linearly with time. When we compare with BBa_K1412924 and BBa_K1412999, we can get that the fluoresent expression intensity of BBa_K1412716 is higher than BBa_K141299 and whike lower than BBa_K1412924.
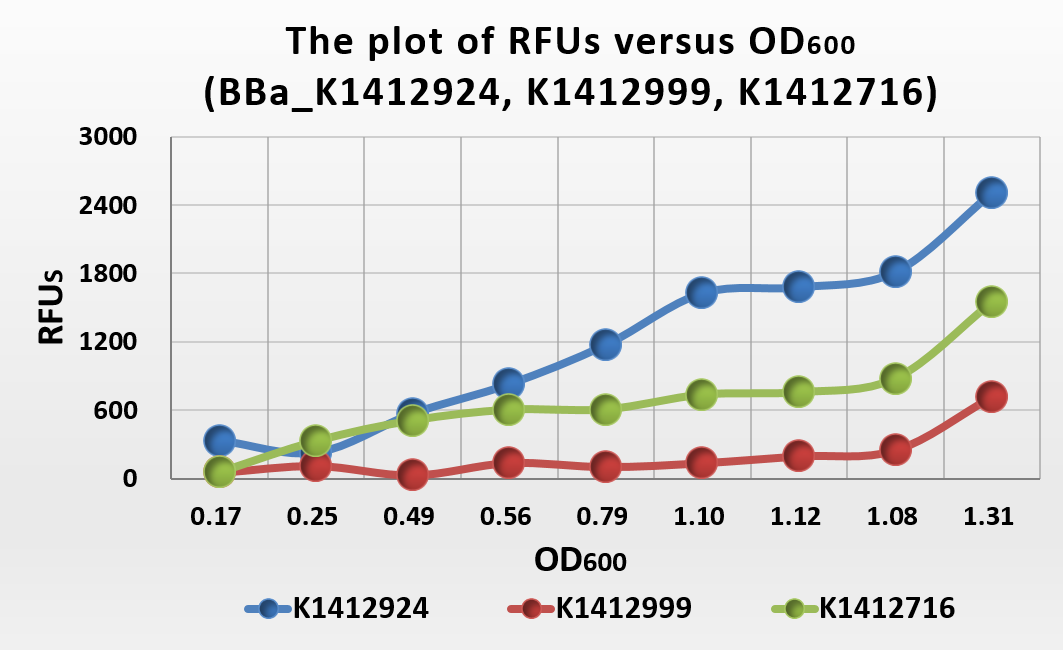
Figure .6 The plot of RFUs versus OD600
From the plot of RFUs versus OD600, we can conclude that RFUs grow linearly with OD600. Because the fluoresent protein expression of each bateria is contain, so when the concentration of bacteria increase, the fluoresent expression increase too. When we compare with BBa_K1412716 and BBa_K1412999, we can get that the fluoresent expression intensity of BBa_K1412716 is higher than BBa_K141299 and whike lower than BBa_K1412924.
Figure 7. The plot of RFUs/OD600 versus time
From the plot of RFUs/OD600 versus time, we know the RFUs/OD600 is a representation of the fluoresent expression intensity of unit bacteria. So we can get that the fluoresent expression intensity of BBa_K1412716 is higher than BBa_K141299 and whike lower than BBa_K1412924.
Protocol
1. Transformed BBa_K1412716 into DH5α competent cells, coated plates, grown in incubator for 12 hrs at 37℃.
2. Inoculate a 5 ml cultures of supplemented LB medium and antibiotic (Kanamycin 50 μg/ml) with single colony from the plate.
3. Cultures were grown in conical flask for 16 hrs at 37℃ with shaking at 200 rpm in the table concentrator.
4. Cultures were diluted 1:100 into three 20 ml fresh LB medium and grown for 3 hrs at 37℃ with shaking at 200 rpm in the table concentrator.
5. Then transfered 650 μl of the culture to a 1.5 ml centrifuge tube, centrifuged and washed twice with phosphate-buffered saline ([http://en.wikipedia.org/wiki/Phosphate_buffered_saline PBS], pH 7.4) to minimize the background fluorescence from the medium.
6. The washed cells were suspended in [http://en.wikipedia.org/wiki/Phosphate_buffered_saline PBS] and diluted to bring the cells into an appropriate concentration range (2–5 times) before taking fluorimeter measurements.
7. Measure the fluorescence and absorbance:
(1)Fluorescence:
- Device: [http://www.moleculardevices.com/systems/microplate-readers/multi-mode-readers/spectramax-m-series-multi-mode-microplate-readers SpectraMax+M5 microplate reader], 96-well plates.
- Wavelengths: 501 nm excitation, 514 nm emission, Auto-cutoff: 515 nm.
(2)OD600 (optical density at 600 nm):
- Device: [http://www.moleculardevices.com/systems/microplate-readers/multi-mode-readers/spectramax-m-series-multi-mode-microplate-readers SpectraMax+M5 microplate reader], 96-well plates.
- Wavelengths: 600 nm absorption.
8. Measure every 30 minutes in the next 4 hrs.
References
[1] [http://journals.aps.org/pre/abstract/10.1103/PhysRevE.82.021911 Bagh, Sangram, Mahuya Mandal, and David R. McMillen. "Minimal genetic device with multiple tunable functions." Physical Review E 82.2 (2010): 021911]
More information, click here: [http://2014.igem.org/Team:XMU-China# XMU-China]
| None |