Difference between revisions of "Part:BBa K500001"
| Line 27: | Line 27: | ||
</html> | </html> | ||
| − | [[File:T-- | + | [[File:T--Uppsala Universitet--MnP-Gel-new without-legend.png|800px|thumb|left|<b>Figure 1: Expression and secretion of Manganese Peroxidase (MnP)</b> <br> |
| + | X-33 <I>P. pastoris</I> cells were transformed with pPICZαB_MnP, induced (i), fractionated into pellets (P) and supernatants (S), and analysed by 10 % SDS-PAGE stained with Coomassie Blue. u, uninduced <br> | ||
| + | Induction bands are visible at approximately 70 kDa for both P and S (red arrows), showing secretion of MnP.]] | ||
| − | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> |
<!-- Add more about the biology of this part here | <!-- Add more about the biology of this part here | ||
Revision as of 22:29, 19 October 2019
lignin degradation 2
Yeast codon optimization , no terminator codon, from Phanerochaete chrysosporium. Synthetized by Geneart Mn peroxidases (MnP) are extracellular hemeproteins first discovered in the white-rot fungus Phanerochaete chrysosporium, and have been virtually detected in all lignin degrading fungi so far studied. The catalytic cycle of MnP is similar to that of other plant and fungal peroxidases
Contribution
Group: iGEM19_Uppsala_Universitet
Author: Jonas Gockel
Summary: We studied the expression of BBa_K500001 in Pichia pastoris under the control of the AOX1 promoter BBa_K3105675 and an N-terminal fused α-secretion factor BBa_K3105674.
To express BBa_K500001, overhang PCR was performed to add complementary regions towards the commercial pPICZαB vector, in which MnP was cloned by gibson assembly. In this vector, the gene is controlled by the AOX1-promoter and is cloned in-frame to an N-terminal α-secretion factor from S. cerevisiae. MnP was expressed in the X-33 wildtype strain of Pichia pastoris, where secretion was observed (Figure 1).
The full expression circuit can be found here:
BBa_K3105682
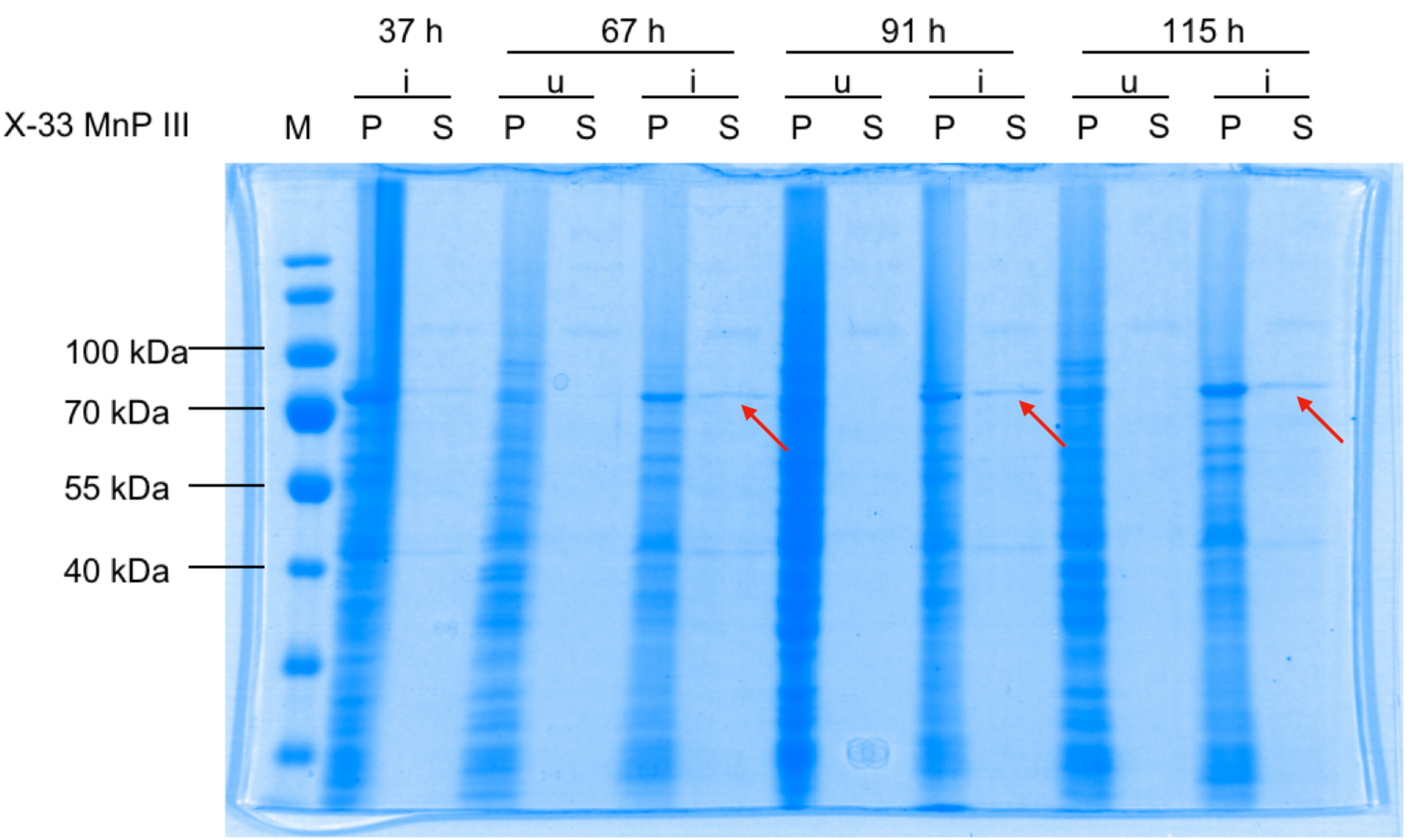
X-33 P. pastoris cells were transformed with pPICZαB_MnP, induced (i), fractionated into pellets (P) and supernatants (S), and analysed by 10 % SDS-PAGE stained with Coomassie Blue. u, uninduced
Induction bands are visible at approximately 70 kDa for both P and S (red arrows), showing secretion of MnP.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 220
Illegal BglII site found at 512 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
