Difference between revisions of "Part:BBa K3182107"
| Line 49: | Line 49: | ||
[[Image:T--Linkoping Sweden--pln1coli.png|300px]] | [[Image:T--Linkoping Sweden--pln1coli.png|300px]] | ||
[[Image:T--Linkoping Sweden--pln1sub.png|300px]] | [[Image:T--Linkoping Sweden--pln1sub.png|300px]] | ||
| − | <b>Figure B.</b> <i>A</i>: CBD-Pln1 2.8 uM was added to an E. coli BL21 (DE3) | + | <b>Figure B.</b> <i>A</i>: CBD-Pln1 2.8 uM was added to an E. coli BL21 (DE3) 0 OD culture, the positive control is water at the same volume as the peptide added. <i>B</i>: Cleaved Pln1 was added to an E. coli BL21 (DE3) 0 OD culture. The positive control contain thrombin and thrombin cleavage buffer with the same volume as Pln1 (40 uL). <i>C</i>: Pln1 was added to a B. subtilis 0 OD culture. The positive control contain thrombin and thrombin cleavage buffer with the same volume as Pln1 (40 uL). |
<br> | <br> | ||
<h2>Antimicrobial activity immobilized and released</h2> | <h2>Antimicrobial activity immobilized and released</h2> | ||
| Line 56: | Line 56: | ||
[[Image:T--Linkoping Sweden--pepplåster.png|335px]] | [[Image:T--Linkoping Sweden--pepplåster.png|335px]] | ||
<br> | <br> | ||
| − | <b>Figure B.</b> | + | <b>Figure B.</b> A cellulose bandage (Epiprotect) incubated with E. coli BL21 (DE3) 0 OD cultures. |
| − | + | ||
Revision as of 18:06, 23 September 2019
Contents
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 653
Illegal BamHI site found at 580 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]
Introduction
pT7-CBDcipA-Pln1
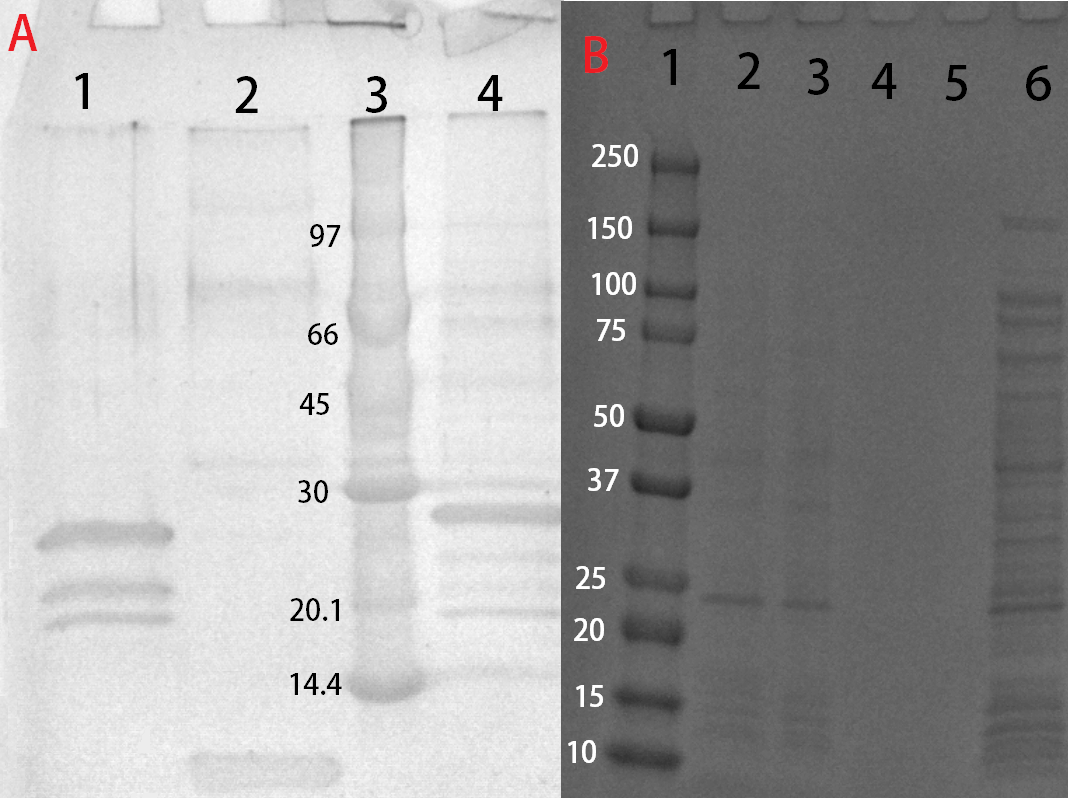
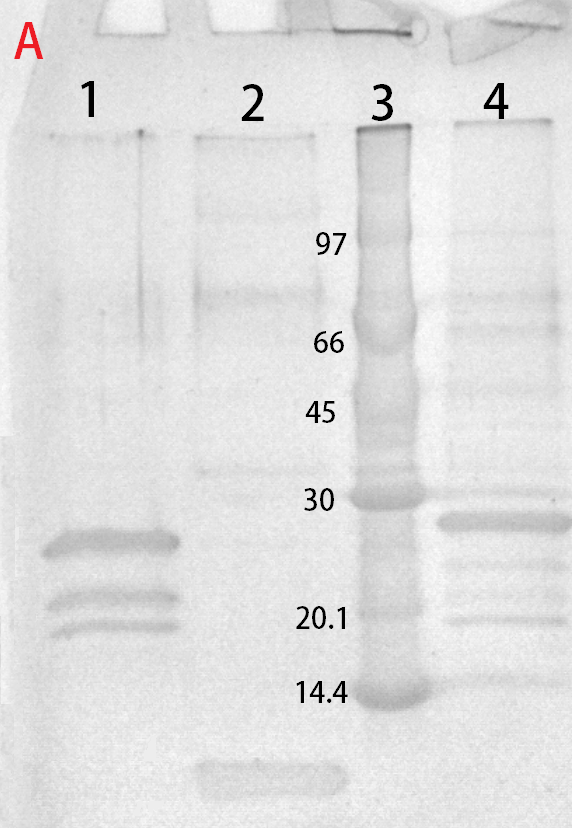
This part consists of a cellulose binding domain (CBD) from Clostridium thermocellum (C. thermocellum) cellulose scaffolding protein (CipA) and is a central part Clostridium thermocellum's cellusome. The CBD was fused to sfGFP in this part to easily track the binding capacities and to test our release mechanism. The CBD-sfGFP were fused using a flexible GS-linker (-GGGGSGGGGS-). A thrombin cleavage site (-LVPRGS-) was added to the end of the linker and its breakage will leave a glycine and serine attached to the N-terminal of the sfGFP fusion protein.
Assembly compabilities
An internal BamHI recognition sequence (RS) has been added to enable changeable fusion proteins. BamHI was chosen because its RS codes for glycine and serine, fitting it to the end of the thrombin site. It is also cost-effective enzyme and is unaffected by methylated DNA.
This part can be used to track purification, measure CBD binding ability and report cleavage at the thrombin site.
CBDcipA crystal structure
Important molecular faces
CBDcipA is composed of a nine-stranded beta sandwich with a jelly roll topology and binds a calcium ion. It further contains conserved residues exposed on the surface which map into two clear surfaces on each side of the molecule. One of faces mainly contains planar strips of aromatic and polar residues which may be the cellulose binding part. Further aspect are unknown and unique with this CBD such as the other conserved residues which are contained in a groove.
The choice of cellulose binding domain
iGEM Linköping 2019 choose CBDcipA due to many other iGEM teams exploring the possibilities of this domain. Our basic design was influenced by iGEM14 Imperial, iGEM15 Edinburgh and iGEM18 Ecuador. Purification and where to place the fusion protein (N- or C-terminal) was determined by studying the former projects. CBDcipA also originates from a thermophilic bacteria which further increases the domains applications.
Expression system
The part has a very strong expression with a T7-RNA-polymerase promotor (BBa_I719005) as well as a 5'-UTR (BBa_K1758100) region which has been shown to further increase expression in E. coli (BBa_K1758106), ([http://www.ncbi.nlm.nih.gov/pubmed/2676996 Olins et al. 1989]), ([http://www.ncbi.nlm.nih.gov/pubmed/23927491 Takahashi et al. 2013]). Both this part and the part were sfGFP was changed for AsPink (BBa_K3182000) showed great expression.

Fusion protein bound to the carbohydrate binding domain
Fused to the CBD is a Lactobacillus plantarum antibacterial peptide. The peptide is 38 amino acids long and has a secondary structure closely resembling membrane proteins. The C-terminal has a high positive charge possibly to find the outer membrane of the target bacteria.
The peptide is designed to battle the Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp. family of pathogens (ESKAPE). ESKAPE is a family(ies) of bacteria which has multiple understrains that has evolved resistance to the most commonly used antibiotics.
Usage and Biology
Expression, purification and protease treatment
Antimicrobial activity in solution
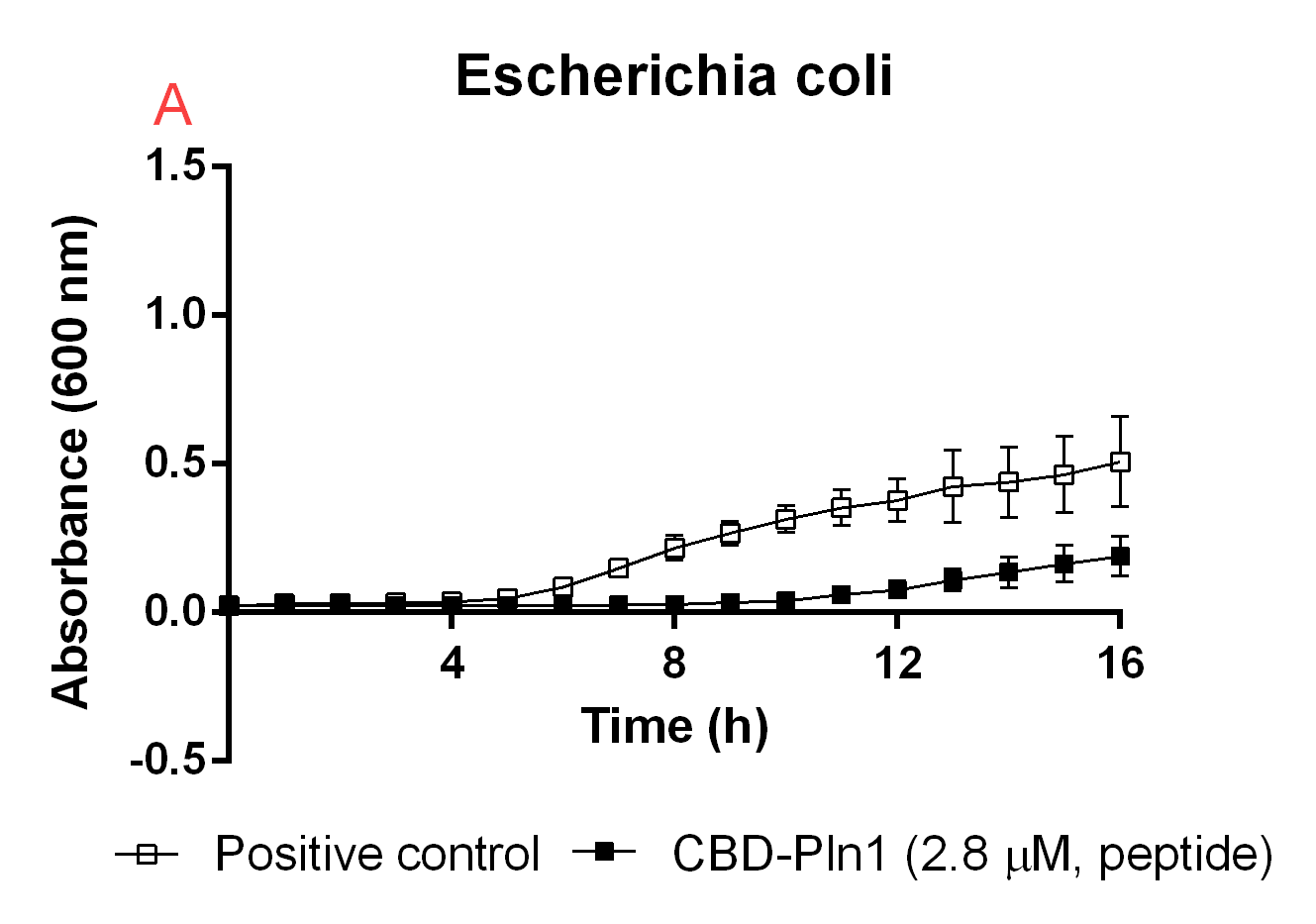
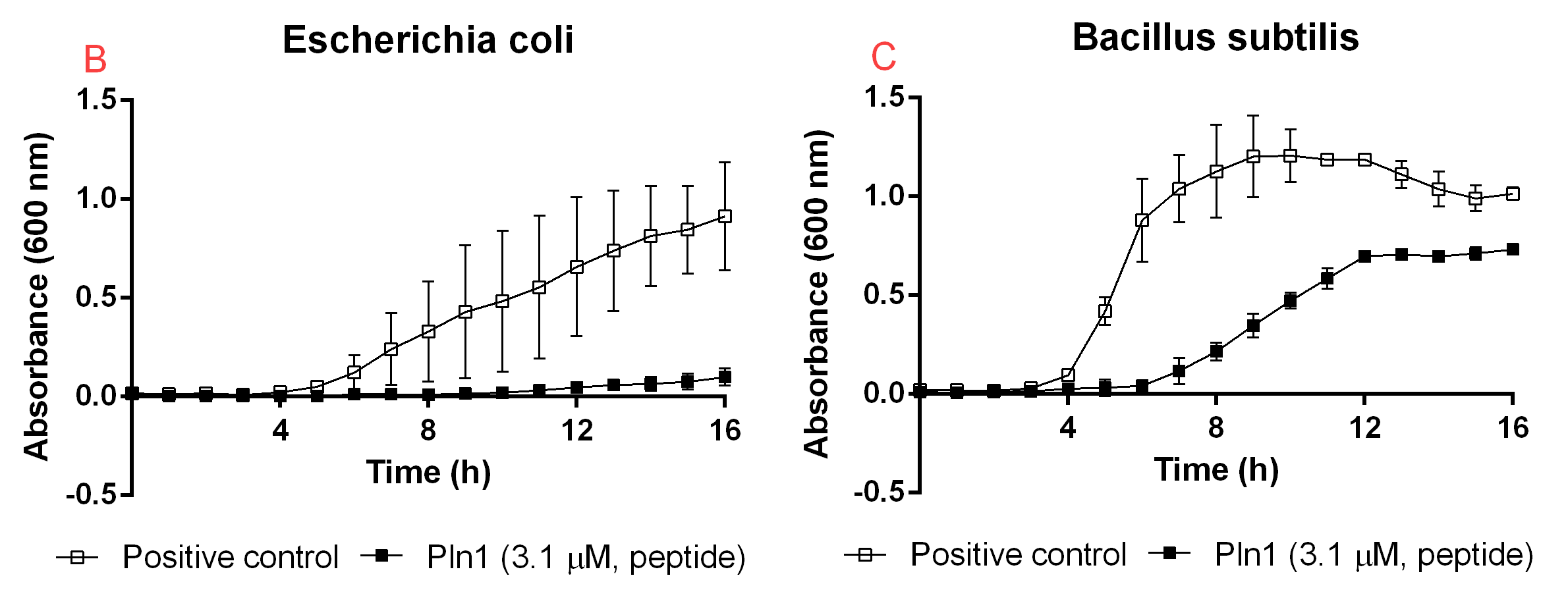
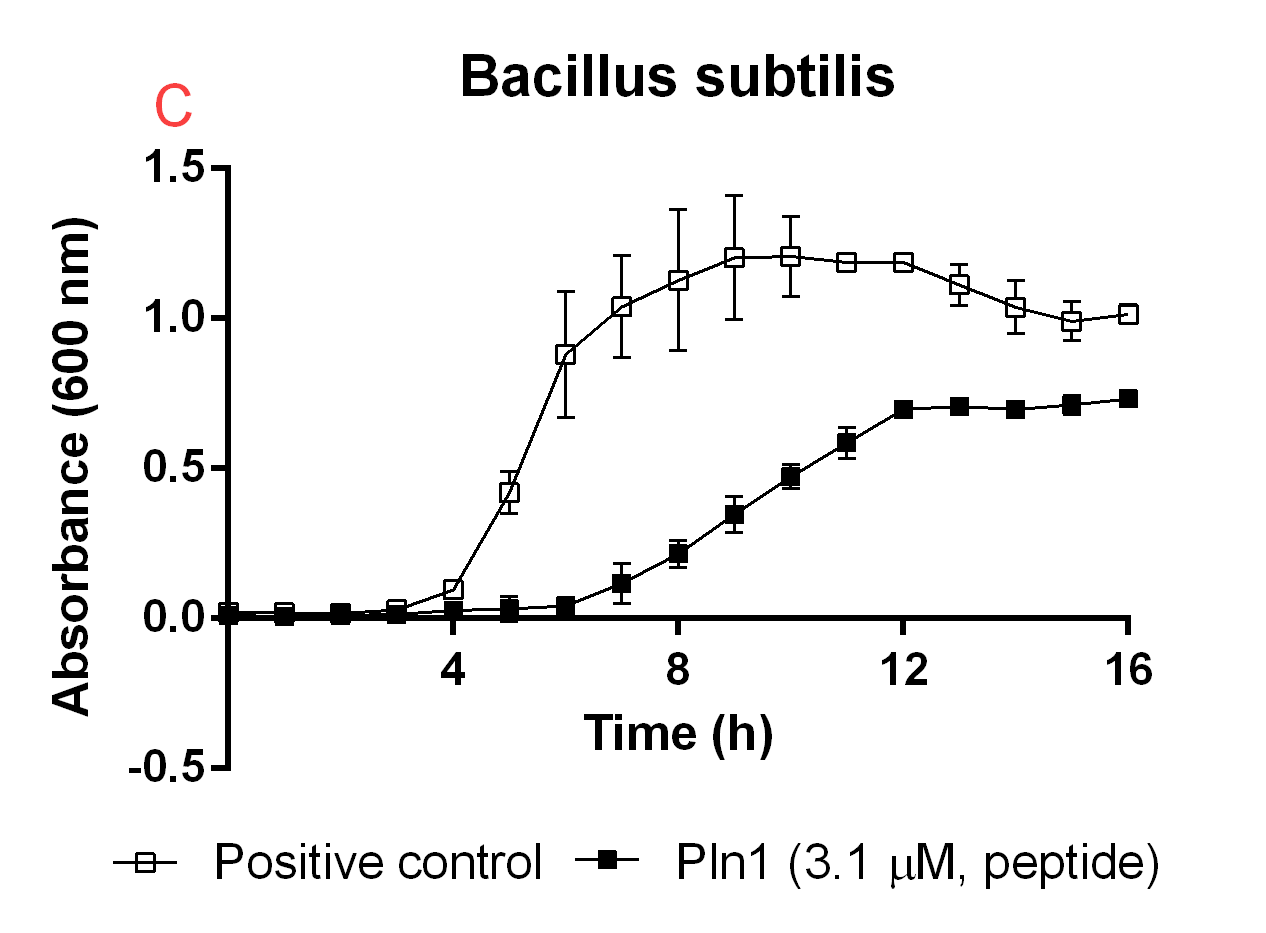 Figure B. A: CBD-Pln1 2.8 uM was added to an E. coli BL21 (DE3) 0 OD culture, the positive control is water at the same volume as the peptide added. B: Cleaved Pln1 was added to an E. coli BL21 (DE3) 0 OD culture. The positive control contain thrombin and thrombin cleavage buffer with the same volume as Pln1 (40 uL). C: Pln1 was added to a B. subtilis 0 OD culture. The positive control contain thrombin and thrombin cleavage buffer with the same volume as Pln1 (40 uL).
Figure B. A: CBD-Pln1 2.8 uM was added to an E. coli BL21 (DE3) 0 OD culture, the positive control is water at the same volume as the peptide added. B: Cleaved Pln1 was added to an E. coli BL21 (DE3) 0 OD culture. The positive control contain thrombin and thrombin cleavage buffer with the same volume as Pln1 (40 uL). C: Pln1 was added to a B. subtilis 0 OD culture. The positive control contain thrombin and thrombin cleavage buffer with the same volume as Pln1 (40 uL).
Antimicrobial activity immobilized and released
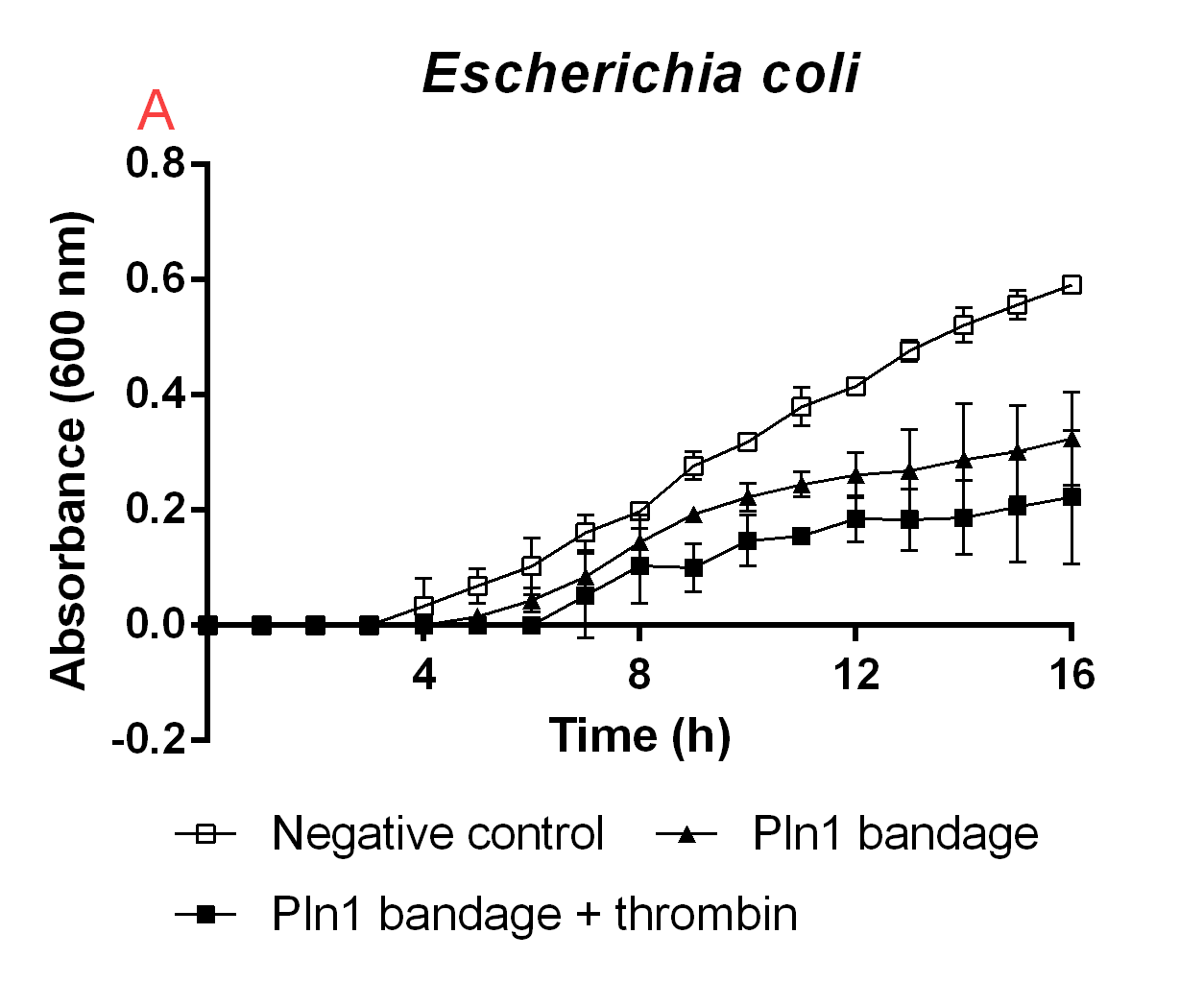
Figure B. A cellulose bandage (Epiprotect) incubated with E. coli BL21 (DE3) 0 OD cultures.