Difference between revisions of "Part:BBa K1618034"
LedaCoelewij (Talk | contribs) |
Nicolapatron (Talk | contribs) m |
||
| Line 2: | Line 2: | ||
<partinfo>BBa_K1618034 short</partinfo> | <partinfo>BBa_K1618034 short</partinfo> | ||
| − | |||
| + | K1618034 (P-35s(K1467101)_TP(K1618037):Rv3030(K1618002)_T-35s(BBa_K1618037)) expresses a putative acyl transferases in plant cells and targets the gene product to the chloroplast. | ||
| − | |||
| + | The parts were assembled using Golden Gate Assembly and the assembly was tested in a binary plasmid vector backbone prior that can replicated in the shuttle chassis t <i>Agrobacterium tumefaciens</i> that transfers the part to plant cells on a T-DNA. For submission to the registry the part was converted to a standard BioBrick for by using the Mo-Flipper [https://parts.igem.org/Part:BBa_K1467400 BBa_K1467400 ] (complete transcriptional unit flipper) made by the NRP-UEA 2014 team. | ||
| − | NRP-UEA 2015 originally used this construct with the aim | + | |
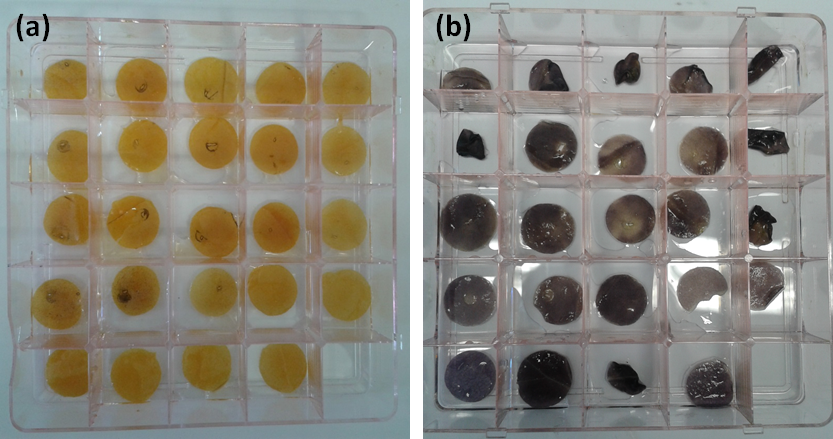
| + | NRP-UEA 2015 originally used this construct with the aim of modifying starch however insufficient quantities of starch were purified from the test system for analysis. Initial tests determines if expression of the enzymes interfered with starch production, a possibility if they compete with starch syntheses of the free ends of glucan chains. Consequently leaves to which the part had been delivered were analysed to predict starch content by iodine staining (Figure 1). Leaves were first put in the dark to remove all existing starch and then starch was allowed to accumulate by allowing the plants access to light. The amount o starch produced in 8 hours was then determined by iodine staining. The expression of this part has no effect on iodine staining but until the starch is analysed for acylation or other modifications, it is impossible to now if the enzyme is functional. An alternate version of this part [https://parts.igem.org/Part:BBa_K1618030, K1618030,] with a C-terminal fluorescent tag indicated that the transit peptide is functioning [https://parts.igem.org/Part:BBa_K1618028 chloroplast transit peptide], and is able to direct the enzyme to the chloroplast where starch is produced. | ||
Revision as of 16:06, 21 September 2015
Acyltransferase Candidate 2 Expression Construct
K1618034 (P-35s(K1467101)_TP(K1618037):Rv3030(K1618002)_T-35s(BBa_K1618037)) expresses a putative acyl transferases in plant cells and targets the gene product to the chloroplast.
The parts were assembled using Golden Gate Assembly and the assembly was tested in a binary plasmid vector backbone prior that can replicated in the shuttle chassis t Agrobacterium tumefaciens that transfers the part to plant cells on a T-DNA. For submission to the registry the part was converted to a standard BioBrick for by using the Mo-Flipper BBa_K1467400 (complete transcriptional unit flipper) made by the NRP-UEA 2014 team.
NRP-UEA 2015 originally used this construct with the aim of modifying starch however insufficient quantities of starch were purified from the test system for analysis. Initial tests determines if expression of the enzymes interfered with starch production, a possibility if they compete with starch syntheses of the free ends of glucan chains. Consequently leaves to which the part had been delivered were analysed to predict starch content by iodine staining (Figure 1). Leaves were first put in the dark to remove all existing starch and then starch was allowed to accumulate by allowing the plants access to light. The amount o starch produced in 8 hours was then determined by iodine staining. The expression of this part has no effect on iodine staining but until the starch is analysed for acylation or other modifications, it is impossible to now if the enzyme is functional. An alternate version of this part K1618030, with a C-terminal fluorescent tag indicated that the transit peptide is functioning chloroplast transit peptide, and is able to direct the enzyme to the chloroplast where starch is produced.
Figure 1: Iodine staining of leaves (a) after a 24 hour dark treatment and (b) after an 8 hour light treatment, where yellow stains mean no starch and black stains mean starch present. Dark treatment was performed so the plant would break down its already existing starch storages. This means that all starch found after the light treatment, when the plant is able to build up its stores again, is indicative on the plants ability to produce starch in the presence of our enzymes. The difference between dark and light treatment is evident, as the majority of the dark treatment is stained yellow, while the light treatments were stained completely black. The fact that the controls and each individual enzyme are indistinguishable in the light treatment suggests that none of the enzymes interfere with the formation of starch.
Unfortunately, we were unable to characterise the starch molecules to visualise if acyl groups were added to the starch. However, with a functioning chloroplast transit peptide, we know our constructs reach the chloroplast where starch is produced. The results above also suggest that if acyl groups are being added to the starch, they are not interfering with the starch production.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 392
Illegal BglII site found at 1138
Illegal XhoI site found at 1342
Illegal XhoI site found at 2486 - 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]