Difference between revisions of "Part:BBa K909007"
| Line 3: | Line 3: | ||
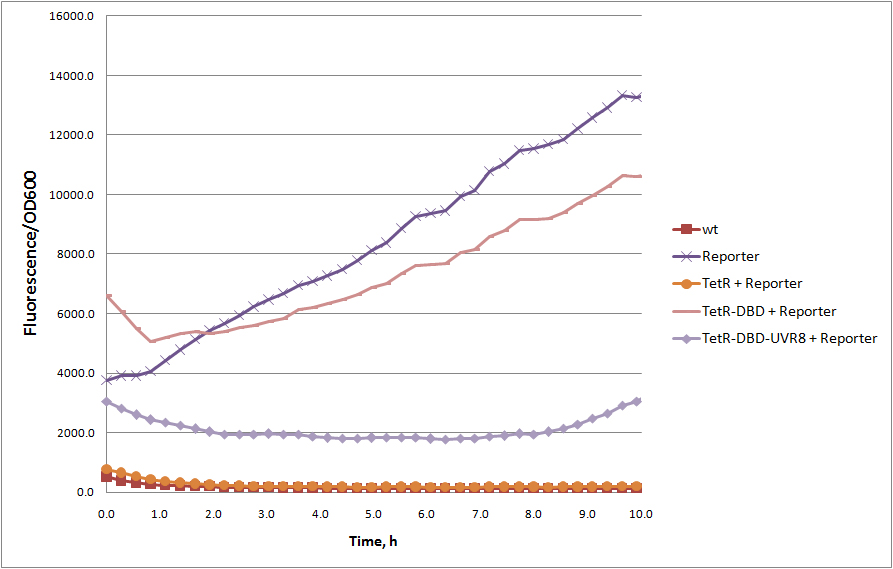
| − | Truncated version of tetracycline repressor TetR, consisting of 1 – 127 amino acids, containing DNA binding domain. The rest of the protein, alpha 8-10 | + | Truncated version of tetracycline repressor TetR, consisting of 1 – 127 amino acids, containing DNA binding domain. The rest of the protein, alpha 8-10 helices responsible for tetR dimerization. Without dimerization domain, tetR-DBD fails to bind TetR responsive promoter efficiently and unable to repress transcription of reporter protein (see figure). However, TetR-DBD fusion with UVR8 protein (<partinfo>BBa_K909008</partinfo>) restores TetR-DBD promoter binding. |
| − | + | ||
| Line 14: | Line 14: | ||
===Usage and Biology=== | ===Usage and Biology=== | ||
| − | We introduced BamHI site at C terminus for an easy in | + | We introduced BamHI site at C terminus of tetR-DBD for an easy in frame fusions with other proteins. This can easily be used as a two hybrid system to detect homo/heterodimers in E. coli, and, in principle, one can use these fusions to turn protein-protein interaction into the repression of transcription. Furthermore, inducible dimerization can lead to a novel switch like behaviors of the system (e.g. [http://2012.igem.org/Team:ETH_Zurich ETH Zurich 2012 team] created a novel UV-B inducible-ON switch). |
| − | + | ||
Revision as of 17:00, 26 September 2012
TetR DNA binding domain containing BamHI site for protein fusions
Truncated version of tetracycline repressor TetR, consisting of 1 – 127 amino acids, containing DNA binding domain. The rest of the protein, alpha 8-10 helices responsible for tetR dimerization. Without dimerization domain, tetR-DBD fails to bind TetR responsive promoter efficiently and unable to repress transcription of reporter protein (see figure). However, TetR-DBD fusion with UVR8 protein (BBa_K909008) restores TetR-DBD promoter binding.
Usage and Biology
We introduced BamHI site at C terminus of tetR-DBD for an easy in frame fusions with other proteins. This can easily be used as a two hybrid system to detect homo/heterodimers in E. coli, and, in principle, one can use these fusions to turn protein-protein interaction into the repression of transcription. Furthermore, inducible dimerization can lead to a novel switch like behaviors of the system (e.g. [http://2012.igem.org/Team:ETH_Zurich ETH Zurich 2012 team] created a novel UV-B inducible-ON switch).
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BamHI site found at 382
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000COMPATIBLE WITH RFC[1000]