Difference between revisions of "Part:BBa J23109:Experience"
(→User Reviews) |
(→Description) |
||
| Line 32: | Line 32: | ||
=====Description===== | =====Description===== | ||
| − | We used | + | We used quantitative real time PCR to examine the expression rate of eight different constitutive promoter constructs of from the parts registry. The reported activities of these promoters are given as the relative fluorescence of these plasmids in strain TG1 ([http://2012.igem.org/Team:Goettingen/Project/Methods#Quantitative_Real-Time_PCR parts registry: part:BBa_J23100]). Promoter constructs were cloned into the vector pSB1C3 and expressed in <i>E.coli BL21DE3</i> grown in LB-media (lysogeny broth). The measurements were performed for each construct and reference as a triplet. Additionally, we concluded H<sub>2</sub>O as negative control to predict possible contamination. For the evaluation of our results, the 2–ΔΔCT (Livak) Method was applied. |
The data were compared to the determined expression rates from the part registry in the following figure. | The data were compared to the determined expression rates from the part registry in the following figure. | ||
You can find detailed information for our qrtPCR approach [http://2012.igem.org/Team:Goettingen/Project/Methods#-.3E_Experimental_design here].<br> | You can find detailed information for our qrtPCR approach [http://2012.igem.org/Team:Goettingen/Project/Methods#-.3E_Experimental_design here].<br> | ||
Revision as of 13:20, 25 September 2012
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_J23109
BBa_K190025 (Planning) constitutive promoter with GVP cluster
BBa_K190031 (Planning) constitutive promoter with fMT
User Reviews
UNIQc3bf8c042e73f11f-partinfo-00000000-QINU
|
•••••
iGEM-Team Goettingen 2012 |
Characterization experiment by qrtPCR on BBa_J23100, BBa_J23104, BBa_J23105, BBa_J23106, BBa_J23109, BBa_J23112, BBa_J23113, BBa_J23114 by iGEM Team Göttingen (by C. Krüger and J. Kampf)DescriptionWe used quantitative real time PCR to examine the expression rate of eight different constitutive promoter constructs of from the parts registry. The reported activities of these promoters are given as the relative fluorescence of these plasmids in strain TG1 ([http://2012.igem.org/Team:Goettingen/Project/Methods#Quantitative_Real-Time_PCR parts registry: part:BBa_J23100]). Promoter constructs were cloned into the vector pSB1C3 and expressed in E.coli BL21DE3 grown in LB-media (lysogeny broth). The measurements were performed for each construct and reference as a triplet. Additionally, we concluded H2O as negative control to predict possible contamination. For the evaluation of our results, the 2–ΔΔCT (Livak) Method was applied.
The data were compared to the determined expression rates from the part registry in the following figure.
You can find detailed information for our qrtPCR approach [http://2012.igem.org/Team:Goettingen/Project/Methods#-.3E_Experimental_design here]. Results & Discussion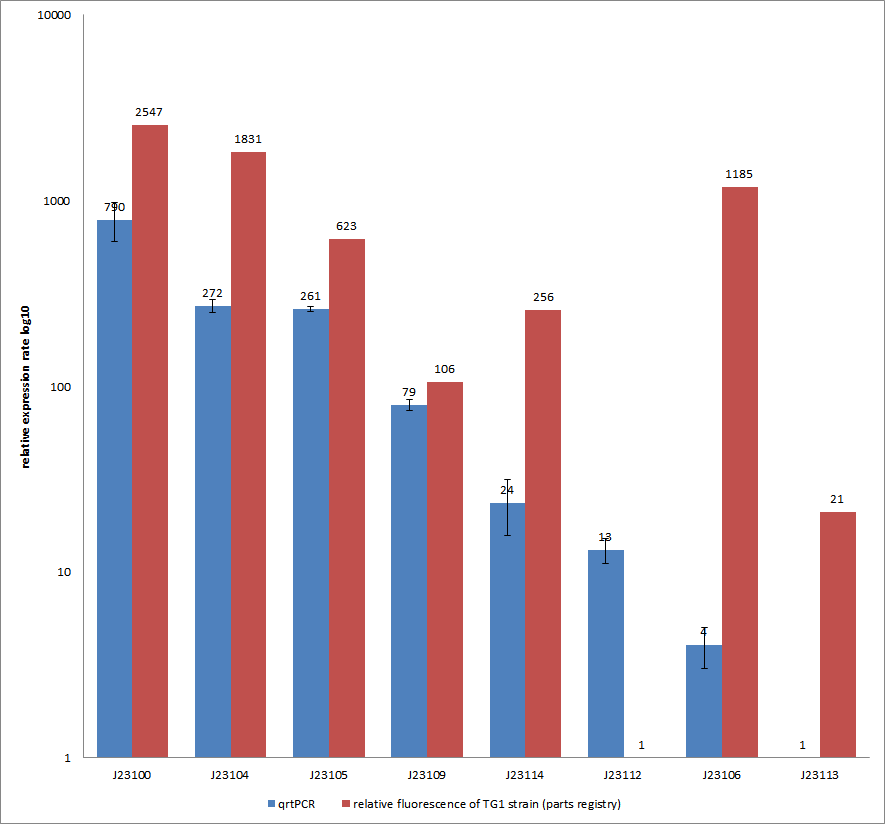 Comparison of relative expression rates of constitutive promoters by qrtPCR and relative fluorescence (parts registry). The blue bar indicates the measured expression rates for our constructs (J23100, J23104, J23105, J23109, J23114, J23113, J23106, J23113) and the red ones those for the literature values represented in the "parts registry" for BBa_J23100. The measurements are illustrated in a logarithmic application. The standard variation was calculated for our measured values (black error bar). Overall, each tested promoter construct indicated differences in expression rates in comparison to values from the “parts registry”. In fact, both data-sets were collected by methods which produce data at different points after gene expression. A common trend was detected for the strongest promoters J23100, J23104, J23105 and J23109 together with the weakest promoters J23112 and J23113. Conspicuously, the promoter J23109 revealed for qrtPCR and for relative fluorescence measurements nearly the same expression rates. The expression rates of J23114 and J23106 indicated massive differences in their expression rates and no common trend with the expression values from “parts registry”. We detected for six of our eight promoters comparable positioning in the ranking of expression rates (see Table, ranking). The promoters characterized as relative strong promoters were also in our case responsible for higher expression rates and the other way around, the promoters characterized as weak ones were in our case responsible for very low expression rates. In the case of promoter J23114 and J23106, the data-sets exhibited a completely different characterization compared to those in the “parts registry”. |
|
•••••
iGEM Groningen 2009 |
We used a number of the constitutive promoter family members for testing our biobricks. The constitutive promoters show the expected level of fluorescence when transformed into E. coli TOP10 cells. Placing parts behind the promoters turned out to be relatively straight forward. We used this part in combination with several biobricks for building our constructs e.g. BBa_I750016 and BBa_K190028 were placed behind the promoters. |
|
•••••
iGEM HKU 2011 |
To start characterizing the promoters, we have performed the red florescence intensity measurements for our selected plasmid in the E.Coli MG1655 strain. The data collected is shown below. It is found that promoter J23106 can lead to a higher expression since the fluorescence intensity per OD600 is the highest, while J23103, J23109, J23116 have relative low expression and fluorescence. As our selected promoters have different strength, thus our team is able to use them to fine tune the protein expression. UNIQc3bf8c042e73f11f-partinfo-00000006-QINU |

