Difference between revisions of "Part:BBa K525304"
JSchwarzhans (Talk | contribs) (→Important parameters) |
JSchwarzhans (Talk | contribs) (→Important parameters) |
||
| Line 30: | Line 30: | ||
|expression of T7 polymerase + IPTG or lactose | |expression of T7 polymerase + IPTG or lactose | ||
|- | |- | ||
| − | |Specific growth rate | + | |Specific growth rate (un-/induced) |
| − | |0.128 h<sup>-1</sup> / 0.094 h<sup>-1</sup> | + | |0.128 h<sup>-1</sup> / 0.094 h<sup>-1</sup> |
|- | |- | ||
| − | |Doubling time | + | |Doubling time (un-/induced) |
| − | |5.41 h / 7.39 h | + | |5.41 h / 7.39 h |
|- | |- | ||
|rowspan="3"|Purification | |rowspan="3"|Purification | ||
Revision as of 19:50, 21 September 2011
Fusion Protein of S-Layer SgsE and mCherry RFP
Fusion protein of S-layer SgsE and mCherry RFP
S-layers (crystalline bacterial surface layer) are crystal-like layers consisting of multiple protein monomers and can be found in various (archae-)bacteria. They constitute the outermost part of the cell wall. Especially their ability for self-assembly into distinct geometries is of scientific interest. At phase boundaries, in solutions and on a variety of surfaces they form different lattice structures. The geometry and arrangement is determined by the C-terminal self assembly-domain, which is specific for each S-layer protein. The most common lattice geometries are oblique, square and hexagonal. By modifying the characteristics of the S-layer through combination with functional groups and protein domains as well as their defined position and orientation to eachother (determined by the S-layer geometry) it is possible to realize various practical applications ([http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.2006.00573.x/full Sleytr et al., 2007]).
Usage and Biology
S-layer proteins can be used as scaffold for nanobiotechnological applications and devices by e.g. fusing the S-layer's self-assembly domain to other functional protein domains. It is possible to coat surfaces and liposomes with S-layers. A big advantage of S-layers: after expressing in E. coli and purification, the nanobiotechnological system is cell-free. This enhances the biological security of a device.
This fluorescent S-layer fusion protein is used to characterize purification methods and the S-layer's ability to self-assemble on surfaces. It is also possible to use the characteristic of mCherry as a pH indicator ([http://pubs.acs.org/doi/abs/10.1021/bm901071b Kainz et al., 2010]).
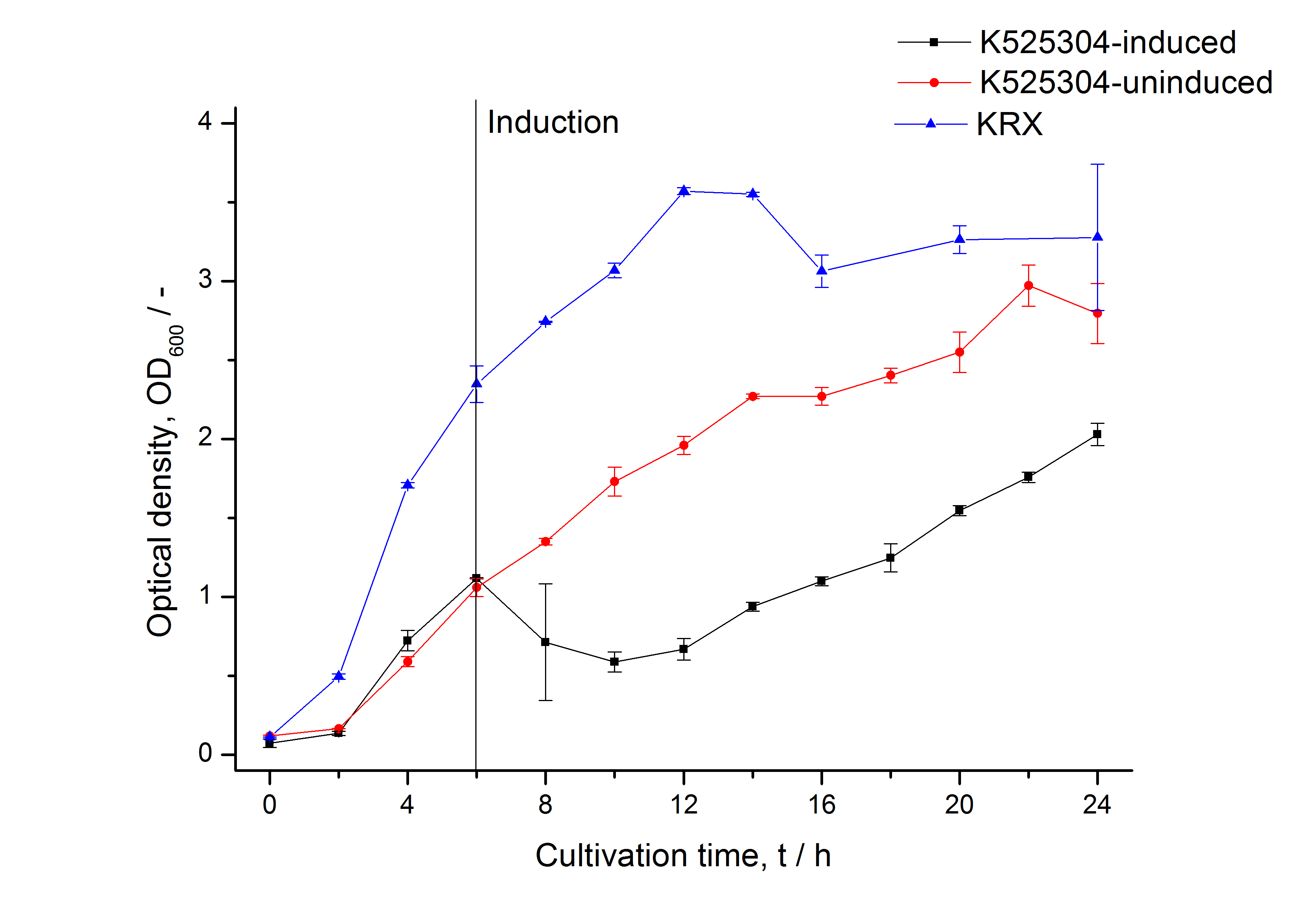
Important parameters
| Experiment | Characteristic | Result |
|---|---|---|
| Expression (E. coli) | Localisation | Inclusion body |
| Compatibility | E. coli KRX and BL21(DE3) | |
| Induction of expression | expression of T7 polymerase + IPTG or lactose | |
| Specific growth rate (un-/induced) | 0.128 h-1 / 0.094 h-1 | |
| Doubling time (un-/induced) | 5.41 h / 7.39 h | |
| Purification | Molecular weight | 109.9 kDa |
| Theoretical pI | 5.70 | |
| Excitation / emission | 587 / 610 nm | |
| Immobilization behaviour | Immobilization time | 4 h |
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21INCOMPATIBLE WITH RFC[21]Illegal BglII site found at 167
Illegal BglII site found at 1022 - 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal NgoMIV site found at 76
Illegal AgeI site found at 3112 - 1000INCOMPATIBLE WITH RFC[1000]Illegal BsaI site found at 1657
Expression in E. coli
The SgsE (K525301) gen was fused with a mCherry RFP (BBa_J18932) using [http://2011.igem.org/Team:Bielefeld-Germany/Protocols#Gibson_assembly Gibson assembly] for characterization.
The SgsE|mCherry RFP fusion protein was overexpressed in E. coli KRX after induction of T7 polymerase by supplementation of 0,1 % L-rhamnose and 1 mM IPTG using the [http://2011.igem.org/Team:Bielefeld-Germany/Protocols/Downstream-processing#Expression_of_S-layer_genes_in_E._coli autinduction protocol] from promega.